A pharmacist and a little science sideblog. "Knowledge belongs to humanity, and is the torch which illuminates the world." - Louis Pasteur
215 posts
Latest Posts by contradictiontonature - Page 5

It’s nearly the season of the witch: time for some cauldron chemistry
Midwives and nurses sometimes came under suspicion because of their specialised knowledge and success - or failure - in treating those who were sick. These healing roles were traditionally taken on by women who, until around the turn of the nineteenth century, were excluded from formal medical training. However, many still practiced medicine in their homes and villages, and what they had learned came from shared knowledge and trial and error, rather than accepted official sources. A medical education might not have been a great help in any case. In the days before germ theory the causes of sickness and the reasons for recovery were not obvious. Any recoveries could be seen as miraculous … or the result of witchcraft.
Treating sickness and disease pre-germ theory was largely guesswork. All sorts of noxious compounds were administered to ailing individuals, and if they produced any effect on the body, be it vomiting, diarrhoea or sweating, it was seen as a good thing – and that was the practice of the so-called professionals. It is not hard to see how images of unofficial healers and herbalists (both men and women) stooping over boiling pots of herbs, roots and who-knows-what, could become a template for the image of a witch, especially when many of the concoctions they produced had such unpleasant effects on their patients.
Having said that, herbalists and traditional healers should not be dismissed as completely ignorant of the medical benefits of some of the plants and poultices they used. Some of the ingredients associated with traditional healing and witches’ potions have been found to be hugely beneficial to medicine once they have been isolated, tested and modified. Science has enabled us to identify the key components of some plants and test them to determine how and when they should be administered safely and effectively. Chemists have modified the structures of some of the compounds to reduce side-effects, make drugs more potent or lower their toxicity.

This year’s Halloween special wraps up the chemistry behind making a mummy: http://wp.me/p4aPLT-26m

Fungal tissues – the fungal mantle around the root tip and the fungal network of tendrils that penetrates the root of plants, or Hartig Net, between Pinus sylvestris plant root cells – in green. Ectomycorrhizal (ECM) fungi help trees tolerate drought and boost the productivity of bioenergy feedstock trees, including poplar and willow.
Via Berkeley Lab: The sclerotia are in the soil!
More: How Fungi Help Trees Tolerate Drought (Joint Genome Institute)

Memory Competition
Most of the brain contains cells that no longer divide and renew. However, the dentate gyrus, nestled within the memory-forming centre of the brain (the hippocampus) is one of the few sites where new cells continue to form throughout life. As a person ages, there is an ever-increasing struggle for these new dentate gyrus neurons (coloured pink) to integrate with existing older neurons (green) because the latter already has well-established connections. This may be why learning and memorisation becomes more difficult as a person gets older. Scientists have now found that by temporarily reducing the number of dendritic spines – branches of neurons that form connections with other neurons – in the mature cells, the new cells have a better chance of functionally integrating. Indeed, in live mice, briefly eliminating dendritic spines boosted the number of integrated new neurons, which rejuvenated the hippocampus and improved the animals’ memory precision.
Written by Ruth Williams
Image courtesy of Kathleen McAvoy
Center for Regenerative Medicine, Massachusetts General Hospital, Boston, MA, USA
Copyright held by original authors
Research published in Neuron, September 2016
You can also follow BPoD on Twitter and Facebook
Dive deep into Episode 05 of #ShelfLife to discover the various technologies that have helped humans map the sky around us for eons.

From sundials to mega-powered modern telescopes, tools for stargazing allow us to understand the universe—and our place within it. Season 2 begins on November 1.


Vexing Virus Shields
Around 36 million people worldwide have HIV. Scientists are trying to develop an HIV vaccine using antibodies, molecules our bodies make to target and tag invading particles. Researchers have found that rabbits exposed to a strain of HIV produce antibodies targeting a specific part of the virus: a hole in its glycan shield. This shield consists of sugar molecules attached to the outside of the virus, protecting it from attack – the hole is thus a gap in its defences. However, although most HIV strains have a hole in their glycan shields, not many strains have one in the same position as was identified, so different antibodies would need to be developed. The image shows how much certain parts of the glycan shield are conserved between different HIV strains – red being 90–100%, and green 50–60%. This is a step towards vaccines, but the shield remains a challenge in vaccine development.
Written by Esther Redhouse White
Image by Sergey Menis, Laura McCoy and James Voss
The Scripps Research Institute, USA and University of Amsterdam, The Netherlands
Image copyright held by original authors
Research published in Cell Reports, August 2016
Published in eLife, June 2016
You can also follow BPoD on Twitter and Facebook

Network Lost
If you believe the theory of six degrees of separation, we’re all connected to each other (and possibly to Kevin Bacon) by common friends and friend-of-friends. It might feel like a small world – in fact, these patterns crops up in all sorts of places. Small world networks connect distant brain cells, and help these lymph nodes (outlined in grey) fight infections. A network of fibroblastic reticular cells (FRCs, red) spreads out inside each node, producing chemicals (green) to support immune cells while they zip around the node gathering antigens – chemical information used to target bacteria. These mouse lymph nodes are treated with different doses of a toxin that destroys FRC networks. A high dose crumples the lymph node in the bottom right. Amazingly, many of these networks repair themselves, showing just how committed immune defences are to keeping their small worlds alive.
Written by John Ankers
Image from work by Mario Novkovic, Lucas Onder and Jovana Cupovic, and colleagues
Institute of Immunobiology, Kantonsspital St. Gallen, St. Gallen, Switzerland
Image originally published under a Creative Commons Licence (BY 4.0)
Published in PLOS Biology, July 2016
You can also follow BPoD on Twitter and Facebook

Different ways to achieve the same goal
via: Science, Critical Thinking and Skepticism


Today is World Anaesthesia Day! Here’s a look at the chemistry behind a range of anaesthetics. More info here and here.


Virus carrying DNA of black widow spider toxin discovered
A tiny virus that may sting like a black widow spider.
That is one of the surprise discoveries made by a pair of Vanderbilt biologists when they sequenced the genome of a virus that attacks Wolbachia, a bacterial parasite that has successfully infected not only black widow spiders but more than half of all arthropod species, which include insects, spiders and crustaceans.
“Discovering DNA related to the black widow spider toxin gene came as a total surprise because it is the first time that a phage – a virus that infects bacteria – has been found carrying animal-like DNA,” said Associate Professor of Biological Sciences Seth Bordenstein. He and Senior Research Specialist Sarah Bordenstein reported the results of their study in a paper titled “Eukaryotic association module in phage WO genomes from Wolbachia” published Oct. 11 in the journal Nature Communications.
Sarah R. Bordenstein, Seth R. Bordenstein. Eukaryotic association module in phage WO genomes from Wolbachia. Nature Communications, 2016; 7: 13155 DOI: 10.1038/ncomms13155
DNA related to black widow spider toxin has been found in a bacterial virus. (iStock)
The oval shape in this electron microphotograph is a Wolbachia bacterium that has infected a Nasonia wasp. The small dots in the bacterium are WO phage particles. The inset shows them at a higher magnification. The white arrows in the inset point to the phage tails. The scale bar in the image is 200 nm and the bar in he inset is 100 nm. (Bordenstein Lab / Vanderbilt)






My new favorite: Solvatofluorescence of Nile Red
Solvatochromism is the ability of a chemical substance to change color due to a change in solvent polarity, so it has different color in different solvents.
Also in some cases, the emission and excitation wavelength both shift depending on solvent polarity, so it fluoresces with different color depending on the solvent what it’s dissolved in. This effect is solvatofluorescence.
On the video the highly solvatochromic organic dye, Nile Red was added to different organic solvents and was diluted with another, different polarity organic solvent. As the polarity of the solution changed, the emitted color from the fluorescent dye also varied as seen on the gifs above and as seen on the video:
To help the blog, donate to Labphoto through Patreon: https://www.patreon.com/labphoto

TODAY IN SCIENCE: Ada Lovelace Day
Ada Lovelace Day is an international celebration of the achievements of women in science, technology, engineering, math, and all related STEM fields.
The celebration is named in honor of English mathematician Augusta Ada King (1815-1852), Countess of Lovelace, known colloquially as Ada Lovelace. Lovelace, the daughter of Lord Byron, is sometimes considered the world’s first computer programmer for the algorithm she wrote for Charles Babbage’s analytical engine, one of the world’s first mechanical computers. Over the years there have been historical disagreements over the extent of Lovelace’s knowledge of the subject and the originality of the work she published in her article, “Sketch of the Analytical Engine, with Notes from the Translator,“ but Babbage himself seemed to dismiss such future claims in his memoir.
Check out the collection Science NetLinks put together for Women’s History Month for related resources to help all students understand the role women historically have played in the history of STEM development and those they play in current STEM fields.
Learn more.
Image Credit: Alfred Edward Chalon [Public domain], via Wikimedia Commons


A special organic dye, Nile Red in different solvents.
From left to right I dissolved equal amounts of Nile Red (a dye) in different solvents. The solvents were: methanol, diisopropyl ether, hexane, n-propanol, tetrahydrofuran, toluene, ethanol, acetone.
Depending on the solvents polarity, the dye dissolved to give different colored solutions (upper image), this is called solvatochromism. It is the ability of a chemical substance to change color due to a change in solvent polarity.
Under UV light, these solutions emitted different colors (bottom pics), this is called solvatofluorescence. The emission and excitation wavelength both shift depending on solvent polarity, so it fluoresces with different color depending on the solvent what it’s dissolved in.
Nile Red is a quite expensive dye, which costs a bit over 1000 USD/gram, therefore I had to make it. The purification of the raw material was posted HERE.
To help the blog, donate to Labphoto through Patreon: https://www.patreon.com/labphoto
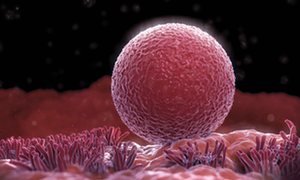
Evidence suggests women’s ovaries can grow new eggs
Scientists have uncovered the first evidence that the human ovary may be able to grow new eggs in adulthood.
If confirmed, the discovery would overturn the accepted view that women are born with a fixed number of eggs and that the body has no capacity to increase this supply. Until now this has been the main constraint on the female reproductive lifespan. The findings, if replicated, would raise the prospect of new treatments to allow older women to conceive and for infertility problems in younger women.
The small study, involving cancer patients, showed that ovarian biopsies taken from young women who had been given a chemotherapy drug had a far higher density of eggs than healthy women of the same age.
Prof Evelyn Telfer, who led the work at the University of Edinburgh, said: “This was something remarkable and completely unexpected for us. The tissue appeared to have formed new eggs. The dogma is that the human ovary has a fixed population of eggs and that no new eggs form throughout life.”
Ovarian biopsies taken from young women who had been given a particular chemotherapy drug showed that the tissue appeared to have formed new eggs. Photograph: Science Picture Co/Getty Images/Science Faction

(Image caption: Antidepressants move G proteins out of lipid rafts in the cell membrane. Credit: Molly Huttner)
Why do antidepressants take so long to work?
An episode of major depression can be crippling, impairing the ability to sleep, work, or eat. In severe cases, the mood disorder can lead to suicide. But the drugs available to treat depression, which can affect one in six Americans in their lifetime, can take weeks or even months to start working.
Researchers at the University of Illinois at Chicago have discovered one reason the drugs take so long to work, and their finding could help scientists develop faster-acting drugs in the future. The research was published in the Journal of Biological Chemistry.
Neuroscientist Mark Rasenick of the UIC College of Medicine and colleagues identified a previously unknown mechanism of action for selective serotonin reuptake inhibitors, or SSRIs, the most commonly prescribed type of antidepressant. Long thought to work by preventing the reabsorption of serotonin back into nerve cells, SSRIs also accumulate in patches of the cell membrane called lipid rafts, Rasenick observed, and the buildup was associated with diminished levels of an important signal molecule in the rafts.
“It’s been a puzzle for quite a long time why SSRI antidepressants can take up to two months to start reducing symptoms, especially because we know that they bind to their targets within minutes,” said Rasenick, distinguished professor of physiology and biophysics and psychiatry at UIC. “We thought that maybe these drugs have an alternate binding site that is important in the action of the drugs to reduce depressive symptoms.”
Serotonin is thought to be in short supply in people with depression. SSRIs bind to serotonin transporters – structures embedded within nerve-cell membranes that allow serotonin to pass in and out of the nerve cells as they communicate with one another. SSRIs block the transporter from ferrying serotonin that has been released into the space between neurons – the synapse – back into the neurons, keeping more of the neurotransmitter available in the synapse, amplifying its effects and reducing symptoms of depression.
Rasenick long suspected that the delayed drug response involved certain signaling molecules in nerve-cell membranes called G proteins.
Previous research by him and colleagues showed that in people with depression, G proteins tended to congregate in lipid rafts, areas of the membrane rich in cholesterol. Stranded on the rafts, the G proteins lacked access to a molecule called cyclic AMP, which they need in order to function. The dampened signaling could be why people with depression are “numb” to their environment, Rasenick reasoned.
In the lab, Rasenick bathed rat glial cells, a type of brain cell, with different SSRIs and located the G proteins within the cell membrane. He found that they accumulated in the lipid rafts over time — and as they did so, G proteins in the rafts decreased.
“The process showed a time-lag consistent with other cellular actions of antidepressants,” Rasenick said. “It’s likely that this effect on the movement of G proteins out of the lipid rafts towards regions of the cell membrane where they are better able to function is the reason these antidepressants take so long to work.”
The finding, he said, suggests how these drugs could be improved.
“Determining the exact binding site could contribute to the design of novel antidepressants that speed the migration of G proteins out of the lipid rafts, so that the antidepressant effects might start to be felt sooner.”
Rasenick already knows a little about the lipid raft binding site. When he doused rat neurons with an SSRI called escitalopram and a molecule that was its mirror image, only the right-handed form bound to the lipid raft.
“This very minor change in the molecule prevents it from binding, so that helps narrow down some of the characteristics of the binding site,” Rasenick said.

The 2016 Nobel Prize in Chemistry is awarded to Jean-Pierre Sauvage, Sir Fraser Stoddart, and Bernard Feringa for the design and production of molecular machines with controllable movements: bit.ly/NobelSci2016

Poison in the Brain: Toxic Structures in Neuronal Nuclei of Alzheimer’s Patients
Spherical structures in the nucleus of nerve cells, so-called nuclear spheres, are suspected to trigger Alzheimer’s disease. A team headed by Dr Thorsten Müller from the research group Cell Signaling in Neurodegeneration has for the very first time demonstrated the presence of the presumably toxic protein aggregates in the human brain. The researchers from Ruhr-Universität Bochum have published their article in the journal Neurobiology of Aging.
The team compared brain samples from Alzheimer’s patients with those of the healthy individuals in the same age group. The result: in the samples taken from Alzheimer’s patients, the number of nuclear spheres was much higher than in those taken from healthy study participants.
Moreover, the group from Bochum analysed in what way nuclear spheres are generated. It was demonstrated in experiments with cell cultures that the amyloid precursor protein (APP) plays a crucial role in this process. APP has long been associated with Alzheimer’s disease. The researchers observed that nuclear sphere generation preferably takes place, if the amyloid precursor protein carries no phosphate group in a specific amino acid. An APP cleavage product, moreover, is contained in the nuclear spheres.
“Extensive nuclear sphere generation in the human Alzheimer’s brain” by Katharina Kolbe, Hassan Bukhari, Christina Loosse, Gregor Leonhardt, Annika Glotzbach, Magdalena Pawlas, Katharina Hess, Carsten Theiss, and Thorsten Müller in Neurobiology of Aging. Published online August 18 2016 doi:10.1016/j.neurobiolaging.2016.08.016
![WATCH: A Macro Timelapse Highlights The Micro Movements Of Spectacularly Colored Coral [video]](https://64.media.tumblr.com/388d126ab9a2d79f43a48c5a471a8b04/tumblr_oe9ygtrlyC1rte5gyo1_500.gif)
![WATCH: A Macro Timelapse Highlights The Micro Movements Of Spectacularly Colored Coral [video]](https://64.media.tumblr.com/77e046f99827b0fc313ab78d09892238/tumblr_oe9ygtrlyC1rte5gyo3_500.gif)
![WATCH: A Macro Timelapse Highlights The Micro Movements Of Spectacularly Colored Coral [video]](https://64.media.tumblr.com/3e50c743bab7ce56ef32f28c9ac9e0d7/tumblr_oe9ygtrlyC1rte5gyo2_500.gif)
![WATCH: A Macro Timelapse Highlights The Micro Movements Of Spectacularly Colored Coral [video]](https://64.media.tumblr.com/787e51d05551b5d9503035887bb0246a/tumblr_oe9ygtrlyC1rte5gyo4_500.gif)
WATCH: A Macro Timelapse Highlights the Micro Movements of Spectacularly Colored Coral [video]

The first of the 2016 science Nobel Prizes was announced earlier today. The prize for Medicine or Physiology was awarded to Yoshinori Ohsumi for his work on the mechanisms behind autophagy. Find out more about his Nobel-winning work with this graphic! http://bit.ly/NobelSci2016

The Nobel prize in medicine has been awarded to a Japanese cell biologist for discoveries on how cells break down and recycle their own components.
Yoshinori Ohsumi, 71, will receive the prestigious 8m Swedish krona (£718,000) award for uncovering “mechanisms for autophagy”, a fundamental process in cells that scientists believe can be harnessed to fight cancer and dementia.
Autophagy is the body’s internal recycling programme - scrap cell components are captured and the useful parts are stripped out to generate energy or build new cells. The process is crucial for preventing cancerous growths, warding off infection and, by maintaining a healthy metabolism, it helps protect against conditions like diabetes.
Dysfunctional autophagy has been linked to Parkinson’s disease, type 2 diabetes, cancer and a host of age-related disorders. Intense research is underway to develop drugs that can target autophagy to treat various diseases.
Speaking to reporters in Tokyo on Monday, Ohsumi said: “As a boy, the Nobel Prize was a dream, but after starting my research, it was out of my picture.”
He said he chose to focus on the cell’s waste disposal system, an unfashionable subject at the time, because he wanted to work on something different.
“I don’t feel comfortable competing with many people, and instead I find it more enjoyable doing something nobody else is doing,” he added. “In a way, that’s what science is all about, and the joy of finding something inspires me.”
Ohsumi, who was in his lab when he received the phone call from Thomas Perlmann, secretary of the Nobel Committee, admitted to being in a “slight state of shock” about the news.
Continue Reading.

(Image caption: A new technique called magnified analysis of proteome (MAP), developed at MIT, allows researchers to peer at molecules within cells or take a wider view of the long-range connections between neurons. Credit: Courtesy of the researchers)
Imaging the brain at multiple size scales
MIT researchers have developed a new technique for imaging brain tissue at multiple scales, allowing them to peer at molecules within cells or take a wider view of the long-range connections between neurons.
This technique, known as magnified analysis of proteome (MAP), should help scientists in their ongoing efforts to chart the connectivity and functions of neurons in the human brain, says Kwanghun Chung, the Samuel A. Goldblith Assistant Professor in the Departments of Chemical Engineering and Brain and Cognitive Sciences, and a member of MIT’s Institute for Medical Engineering and Science (IMES) and Picower Institute for Learning and Memory.
“We use a chemical process to make the whole brain size-adjustable, while preserving pretty much everything. We preserve the proteome (the collection of proteins found in a biological sample), we preserve nanoscopic details, and we also preserve brain-wide connectivity,” says Chung, the senior author of a paper describing the method in the July 25 issue of Nature Biotechnology.
The researchers also showed that the technique is applicable to other organs such as the heart, lungs, liver, and kidneys.
The paper’s lead authors are postdoc Taeyun Ku, graduate student Justin Swaney, and visiting scholar Jeong-Yoon Park.
Multiscale imaging
The new MAP technique builds on a tissue transformation method known as CLARITY, which Chung developed as a postdoc at Stanford University. CLARITY preserves cells and molecules in brain tissue and makes them transparent so the molecules inside the cell can be imaged in 3-D. In the new study, Chung sought a way to image the brain at multiple scales, within the same tissue sample.
“There is no effective technology that allows you to obtain this multilevel detail, from brain region connectivity all the way down to subcellular details, plus molecular information,” he says.
To achieve that, the researchers developed a method to reversibly expand tissue samples in a way that preserves nearly all of the proteins within the cells. Those proteins can then be labeled with fluorescent molecules and imaged.
The technique relies on flooding the brain tissue with acrylamide polymers, which can form a dense gel. In this case, the gel is 10 times denser than the one used for the CLARITY technique, which gives the sample much more stability. This stability allows the researchers to denature and dissociate the proteins inside the cells without destroying the structural integrity of the tissue sample.
Before denaturing the proteins, the researchers attach them to the gel using formaldehyde, as Chung did in the CLARITY method. Once the proteins are attached and denatured, the gel expands the tissue sample to four or five times its original size.
“It is reversible and you can do it many times,” Chung says. “You can then use off-the-shelf molecular markers like antibodies to label and visualize the distribution of all these preserved biomolecules.”
There are hundreds of thousands of commercially available antibodies that can be used to fluorescently tag specific proteins. In this study, the researchers imaged neuronal structures such as axons and synapses by labeling proteins found in those structures, and they also labeled proteins that allow them to distinguish neurons from glial cells.
“We can use these antibodies to visualize any target structures or molecules,” Chung says. “We can visualize different neuron types and their projections to see their connectivity. We can also visualize signaling molecules or functionally important proteins.”
High resolution
Once the tissue is expanded, the researchers can use any of several common microscopes to obtain images with a resolution as high as 60 nanometers — much better than the usual 200 to 250-nanometer limit of light microscopes, which are constrained by the wavelength of visible light. The researchers also demonstrated that this approach works with relatively large tissue samples, up to 2 millimeters thick.
“This is, as far as I know, the first demonstration of super-resolution proteomic imaging of millimeter-scale samples,” Chung says.
“This is an exciting advance for brain mapping, a technique that reveals the molecular and connectional architecture of the brain with unprecedented detail,” says Sebastian Seung, a professor of computer science at the Princeton Neuroscience Institute, who was not involved in the research.
Currently, efforts to map the connections of the human brain rely on electron microscopy, but Chung and colleagues demonstrated that the higher-resolution MAP imaging technique can trace those connections more accurately.
Chung’s lab is now working on speeding up the imaging and the image processing, which is challenging because there is so much data generated from imaging the expanded tissue samples.
“It’s already easier than other techniques because the process is really simple and you can use off-the-shelf molecular markers, but we are trying to make it even simpler,” Chung says.
Phenyllithium or lithobenzene is an organometallic agent with the empirical formula C6H5Li. Crystalline phenyllithium is colorless; however, solutions of phenyllithium are various shades of brown or red depending on the solvent. It is a highly air and moisture sensitive compound, that could be easily decomposed by any protic solvent.

In this case it was a byproduct of a synthesis of an organophosphorous compound and it was only present in a LOW concentration, therefore it was safe to decompose it by simply adding cold water. It’s important to note that it could be dangerous to decompose organometallic compounds by simply adding water. Also, in this case, the highly toxic BENZENE was the product of this reaction, what should be handled with care.


COLORS OF CHEMISTRY
The bright colors of chemistry fascinate people of all ages. Hriday Bhattacharjee, a Ph.D. student in the lab of Jens Mueller at the University of Saskatchewan, assembled this showcase from compounds he prepared as well as from some synthesized by the undergraduate students he teaches. Organometallic and inorganic chemistry—the study of molecules like these that involve metal atoms—is especially colorful.
The table below the picture indicates the chemicals seen in the photo.
Submitted by Hriday Bhattacharjee
Do science. Take pictures. Win money: Enter our photo contest.

We Just Moved One Step Closer to Understanding (and Defeating) Alzheimer’s






Why do flowers do the thing they do?
Flowers are the reproductive organs of plants. When pollinated, flowers develop into fruits containing seeds. However, producing flowers, fruits, and seeds is not easy. Plants devote lots of resources and energy to grow these specialized organs. Thus, plants tend to synchronize their efforts with a time of year when conditions are best for reproductive success and survival.
“Annuals” are plants that grow from seed, flower, and die in one year. Since annuals need to grow leaves and stems before they flower, most annuals won’t mature enough to flower until mid-summer or later.
“Winter annuals” get a jump-start on reproduction by germinating from seeds in the fall, over-wintering as rosettes of leaves and storing energy which allows them to flower early in the spring.
“Perennial” plants can live for many years and flower multiple times. Perennials have evolved many different flowering strategies. Most flower in mid- to late summer after they have had time to accumulate the resources needed to produce seeds each year. Others, such as early forest wildflowers, grow for only a short while, blooming before the trees above them leaf out, starving them of light. These plants store energy in underground roots or stems, allowing them to flower early and quickly.
The evolution of such diverse flowering strategies is good for plants that otherwise would have to compete for the same resources at the same time. Its also is nice for us, as we get to enjoy flowers brightening the landscape throughout the growing season.
Giffed by: rudescience From: this video

Finally got the pure Nile Red in solution, just need to evaporate to get the pure dye.
Interesting fact: Nile Red is a solvatochromic dye. What does this mean? Solvatochromism is the ability of a chemical substance to change color due to a change in solvent polarity, so it has different color in different solvents. Also its emission and excitation wavelength both shift depending on solvent polarity, so it fluoresces with with different color depending on the solvent what it’s dissolved in.
In this case it was dissolved in dichloromethane.




Next week I’ll give a presentation on the Researchers Night at Eötvös Loránd University, Hungary with the title: “Chemistry of light and the light of chemistry”.
During this presentation one of my favorite dyes will be also presented: Nile Red. However, just as usual, the 1000 USD/gram price was a bit over our budget, so I had to make it.
The raw product was contaminated with a few impurities, but a fast purification, by simple filtering the mixture through a short column helped a lot and ended up with a +95% pure product.
At first I concentrated the product from a dilute solution on the column as seen on the first pics. It’s interesting to see, that it has a different fluorescence in solution (faint orange fluorescent) and while it’s absorbed on the solid phase (pink, highly fluorescent).
After all the product was on the solid phase, I added another solvent and washed down the pure, HIGHLY FLUORESCENT product. Everything else, what was mainly products of side reactions, stuck at the top of the column as seen on the second pics and the gifs.
Also here is a video from the whole process in HD: https://youtu.be/W0Lk5jkd_B0
Neuroscientists’ Study Sheds Light on How Words Are Represented in the Brain
Reading is a relatively modern and uniquely human skill. For this reason, visual word recognition has been a puzzle for neuroscientists because the neural systems responsible for reading could not have evolved for this purpose. “The existence of brain regions dedicated to reading has been fiercely debated for almost 200 years,” said Avniel Ghuman, an assistant professor in the University of Pittsburgh Department of Neurological Surgery. “Wernicke, Dejerine, and Charcot, among the most important and influential neurologists and neuroscientists of the 19th century, debated whether or not there was a visual center for words in the brain.”

In recent years, much of this debate has centered on the left mid-fusiform gyrus, which some call the visual word form area. A recent study by Pitt neuroscience researchers addresses this debate and sheds light on our understanding of the neurobiology of reading.
In a study published July 19 in the Proceedings of the National Academy of Sciences, Ghuman, Elizabeth Hirshorn of Pitt’s Learning Research and Development Center (LRDC), and colleagues from the Department of Psychology and Center for the Neural Basis of Cognition used direct neural recordings and brain stimulation to study the role of the visual word form area in reading in four epileptic patients. The patients chose surgical treatment for their drug-resistant epilepsy and volunteered to participate in the research study. As part of the surgical treatment, neurosurgeons implanted electrodes in the patients’ visual word form area, providing an unprecedented opportunity to understand how the brain recognizes printed words.
First, painless electrical brain stimulation was used through the electrodes to disrupt the normal functioning of the visual word form area, which adversely affected the patients’ ability to read words. One patient dramatically misperceived letters, and another felt that there were words and parts of words present that were not in what she was reading. Stimulation to this region did not disrupt their ability to name objects or faces. A brief video of the stimulation can be seen here.
In addition to stimulating through these electrodes, the activity from the area was recorded while the patients read words. Using techniques from machine learning to analyze the brain activity that evolved over a few hundred milliseconds from this region, the researchers could tell what word a patient was reading at a particular moment. This suggests that neural activity in the area codes knowledge about learned visual words that can be used to discriminate even words that are only one letter different from one another (for example, “hint” and “lint”).
“This study shows that the visual word form area is exquisitely tuned to the fine details of written words and that this area plays a critical role in refining the brain’s representation of what we are reading. The disrupted word and letter perception seen with stimulation provides direct evidence that the visual word form area plays a dedicated role in skilled reading,” said Hirshorn. “These results also have important implications for understanding and treating reading disorders. The activity in the visual word form area, along with its interactions with other brain areas involved in language processing, could be a marker for proficient reading. Having a better understanding of this neural system could be critical for diagnosing reading disorders and developing targeted therapies.”
“It is exciting that with modern brain-recording techniques and advanced analysis methods, we are finally able to start answering questions about the brain and the mind that people have asked for centuries and contribute to our understanding of reading disorders,” said Ghuman.

Swarms of magnetic bacteria could be used to deliver drugs to tumors
Researchers funded in part by the National Institute of Biomedical Imaging and Bioengineering (NIBIB) have recently shown that magnetic bacteria are a promising vehicle for more efficiently delivering tumor-fighting drugs. They reported their results in the August 2016 issue of Nature Nanotechnology.
Ouajdi Felfoul, Mahmood Mohammadi, Samira Taherkhani, Dominic de Lanauze, Yong Zhong Xu, Dumitru Loghin, Sherief Essa, Sylwia Jancik, Daniel Houle, Michel Lafleur, Louis Gaboury, Maryam Tabrizian, Neila Kaou, Michael Atkin, Té Vuong, Gerald Batist, Nicole Beauchemin, Danuta Radzioch, Sylvain Martel. Magneto-aerotactic bacteria deliver drug-containing nanoliposomes to tumour hypoxic regions. Nature Nanotechnology, 2016; DOI: 10.1038/nnano.2016.137
Illustration showing magnetic bacteria delivering drugs to a tumor. Credit: NanoRobotics Laboratory, Polytechnique Montreal

