A pharmacist and a little science sideblog. "Knowledge belongs to humanity, and is the torch which illuminates the world." - Louis Pasteur
215 posts
Latest Posts by contradictiontonature - Page 6

(Image caption: The synapses of pyramid cells in the cerebral cortex form functional groups. Some of the related synapses are shown in green in the reconstruction. Credit: © MPI of Neurobiology / Scheuss)
Neurons form synapse clusters
The cerebral cortex resembles a vast switchboard. Countless lines carrying information about the environment, for example from the sensory organs, converge in the cerebral cortex. In order to direct the flow of data into meaningful pathways, the individual pyramidal cells of the cerebral cortex act like miniature switchboard operators. Each cell receives information from several thousand lines. If the signals make sense, the line is opened, and the information is relayed onward. Scientists at the Max Planck Institute of Neurobiology in Martinsried have now shown for the first time that contact points between specific neuron types are clustered in groups on the target neuron. It is probable that signals are coordinated with each other in this way to make them more “convincing”.
The cells of the cerebral cortex have a lot to do. They process various types of information depending on the area in which they are located. For example, signals from the retina arrive in the visual cortex, where, among other things, the motion of objects is detected. The pyramidal cells of the cerebral cortex receive information from other cells through thousands of contact points called synapses. Depending on where, how many and how often synapses are activated, the cell relays the signal onward – or not.
Information is passed on in the form of electrical signals. The neurobiologists were able to measure these signals at various contact points of the neuron. “The exciting thing is that the signals that a cell receives from, say, ten simultaneously active synapses can be greater than the sum of the signals from the ten individual synapses,” says Volker Scheuss, summarizing the basis of his recently published study. “However, until now it was unclear whether this phenomenon can be explained by a specific arrangement of synapses on pyramidal cells.”
By combining modern methods, the neurobiologists in Tobias Bonhoeffer’s Department have analysed the arrangement of synapses. They were able to selectively activate a specific type of pyramid cell in brain slices from mice using optogenetics. Thanks to simultaneous “calcium imaging”, they were then able to observe and record the activity of individual synapses under a two-photon microscope. In this way, they succeeded in showing for the first time how synapses are arranged with respect to each other.
The result of such synapse mapping analysed with a newly developed algorithm was clear: The synapses of pyramidal cells form clusters consisting of 4 to 14 synapses arranged within an area of less than 30 micrometres along the dendrite. “The existence of these clusters suggests that the synapses interact with each other to control the strength of the combined signal,” explains Onur Gökçe, author of the study. This is the first anatomical explanation for the disproportionate strength of clustered synapse signals in comparison to the individual signals – a finding known from activity measurements. The observation in layer 5 pyramidal cells was of particular interest, as the activity of these cells oscillates synchronously. “This rhythmic activity, which probably influences the processing of visual information, could synchronously activate synapse clusters, thus boosting the overall signal received,” says Scheuss.

IN THE MIX
Matti Koivisto, an undergraduate student working in the laboratory of Kari Haajanen at Turku University of Applied Sciences, designs solutions similar to this one to detect the bacteria Escherichia coli. He uses a dye called rhodamine 6G, which has a strong orange color when dissolved in ethanol. In solution, the dye separates into negatively and positively charged ions, the latter of which are largely responsible for the dye’s color. Negatively charged molecules on the outer membranes of E. coli attract the dye’s positive ions. This interaction causes the dye to change color to a pinkish hue, and the level of color change allows Koivisto to gauge how many of the bacteria are present.
Submitted by Matti Koivisto
Do science. Take photos. Make money: Enter our monthly photo contest here for your chance to win!
Related C&EN content:
How dyes in mouse feces help track insects
Using an ink-jet printer to simplify analytical chemistry
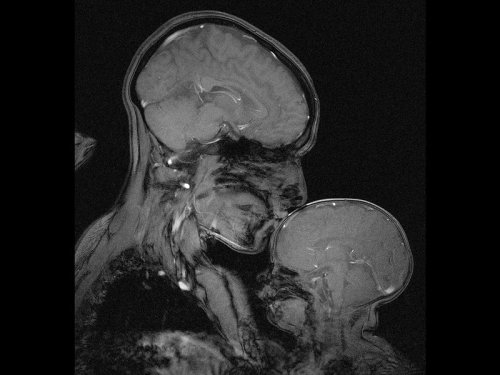
Neuroscientist captures an MRI of a mother and child
Professor Rebecca Saxe (MIT) has taken the first ever MR image of a mother and child.
“This picture is an MR image of a mother and a child that I made in my lab at MIT. You might see it as sweet and touching… an image of universal love. We can’t see clothes or hairstyles or even skin colour. From what we do see, the biology and the brains, this could be any mother and child or even father and child at any time and place in history; having an experience that any human can recognise.
Or you might see it as disturbing, a reminder that our human bodies are much too fragile as houses for ourselves. MRI’s are usually medical images and often bad news. Each white spot in that picture is a blood vessel that could clog, each tiny fold of those brains could harbour a tumour. The baby’s brain maybe looks particularly vulnerable pressed against the soft thin shell of its skull.
I see those things, universal emotions and frightening fragility but I also see one of the most amazing transformations in biology.”
Quotes have been taken from a TEDx talk given by Professor Saxe explaining the story behind the above picture.

Genes to Brains
Over the years scientists have carefully mapped the brain, figuring out which regions perform different functions. Techniques such as functional MRI can show exactly which parts are active when people are doing all kinds of other tasks. Detailed microscopy and brain-scanning studies have traced the intricate network of connections between nerve cells in the brain, revealing the inner wiring of this powerful biological computer. But until now, nobody has tried to link patterns of gene activity into this functional and structural information. For the first time, researchers have generated this map of the brain, with each colour highlighting a particular group of genes that seem to be linked to that region. There are many variations in human genes that can influence traits and conditions affecting the brain, such as intelligence or autism, and this is the first step towards figuring out exactly how these genetic variations might exert their effects.
Written by Kat Arney
Image from work by Qian Peng and colleagues
Department of Human Biology, J. Craig Venter Institute, and Multimodal Imaging Laboratory, Department of Radiology, University of California San Diego, La Jolla, CA, USA
Image originally published under a Creative Commons Licence (BY 4.0)
Published in PLOS Genetics, July 2016
You can also follow BPoD on Twitter and Facebook
Toxic Alzheimer’s Protein Spreads Through Brain Via Extracellular Space
A toxic Alzheimer’s protein can spread through the brain—jumping from one neuron to another—via the extracellular space that surrounds the brain’s neurons, suggests new research from Karen Duff, PhD, and colleagues at Columbia University Medical Center.

(Image caption: Orange indicates where tau protein has traveled from one neuron to another. Credit: Laboratory of Karen Duff, PhD)
The spread of the protein, called tau, may explain why only one area of the brain is affected in the early stages of Alzheimer’s but multiple areas are affected in later stages of the disease.
“By learning how tau spreads, we may be able to stop it from jumping from neuron to neuron,” says Dr. Duff. “This would prevent the disease from spreading to other regions of the brain, which is associated with more severe dementia.”
The idea the Alzheimer’s can spread through the brain first gained support a few years ago when Duff and other Columbia researchers discovered that tau spread from neuron to neuron through the brains of mice.
In the new study, lead scientist Jessica Wu, PhD, of the Taub Institute discovered how tau travels by tracking the movement of tau from one neuron to another. Tau, she found, can be released by neurons into extracellular space, where it can be picked up by other neurons. Because tau can travel long distances within the neuron before its release, it can seed other regions of the brain.
“This finding has important clinical implications,” explains Dr. Duff. “When tau is released into the extracellular space, it would be much easier to target the protein with therapeutic agents, such as antibodies, than if it had remained in the neuron.”
A second interesting feature of the study is the observation that the spread of tau accelerates when the neurons are more active. Two team members, Abid Hussaini, PhD, and Gustavo Rodriguez, PhD, showed that stimulating the activity of neurons accelerated the spread of tau through the brain of mice and led to more neurodegeneration.
Although more work is needed to examine whether those findings are relevant for people, “they suggest that clinical trials testing treatments that increase brain activity, such as deep brain stimulation, should be monitored carefully in people with neurodegenerative diseases,” Dr. Duff says.
DNA from the deep? Antikythera shipwreck yields ancient human bones
Death, when it came, was sudden and cruel. The individual, either a crew member or passenger, was trapped on board when the huge ship foundered. Dashed on the rocks, the vessel slid beneath the waves, tumbled down an undersea cliff, and swiftly became buried in sediment on the seabed.
Now, more than 2,000 years later, archaeologists have recovered the bones of the individual they now call Pamphilos. Thought to be a man in his late teens to early 20s, he was on the ship sailing from Asia Minor to Rome when disaster struck off the tiny Greek island of Antikythera between Crete and the Peloponnese.
The brilliant colors of a soap film reveal the fluid’s thickness, thanks to a process known as thin film interference. The twisting flow of the film depends on many influences: gravity pulls down on the liquid and tends to make it drain away; evaporation steals fluid from the film; local air currents can push or pull the film; and the variation in the concentration of molecules – specifically the surfactants that stabilize the film – will change the local surface tension, causing flow via the Marangoni effect. Together these and other effects create the dancing turbulence captured above. (Video credit: A. Filipowicz)

Clear as Day
The more light your eyes can take in, the better the picture you see, and the lens at the front of your eye is transparent to help this. Most body cells contain lots of membranes – they have important roles like manufacturing cellular components, but they scatter light and aren’t transparent. Cells in the lens become transparent by losing all but their most vital internal membranes as they develop and move towards the middle of the lens: the central cells (shown here in a chick’s eye) are flatter, with rounder nuclei (blue). It wasn’t known how the membranes were lost until recently, when scientists discovered a structure called the excisosome. This forms inside cells and breaks down the membranes, possibly by stripping them apart into the proteins and lipids they’re made of. Current research implies that excisosomes form in the lenses of all animals, helping us understand how our eyes develop.
Written by Esther Redhouse White
Image from work by M.Joseph Costello and colleagues
Department of Cell Biology and Physiology, University of North Carolina, Chapel Hill, NC, USA
Image originally published under a Creative Commons Licence (BY 4.0)
Published in PLOS One, August 2016
You can also follow BPoD on Twitter and Facebook
One of the smoothest, most beautiful color changes I’ve ever seen.
The reaction is methoxymethyl deprotection of one of my agonists with concentrated HCl in acetonitrile as my solvent. The color change doesn’t happen in THF!

(Image caption: The brain of a fruit fly contains many different regions responsible for processing sight, smell and taste in addition to regions for controlling movement. This image shows the results of a new method which automatically identifies these brain regions. Each color represents a different brain region. The authors used this method to discover specific areas involved in processing of visual information in the fly. The technique could also be used to refine our understanding of vertebrate brains)
Identifying Brain Regions Automatically
Using the example of the fruit fly, a team of biologists led by Prof. Dr. Andrew Straw has identified patterns in the genetic activity of brain cells and taken them as a basis for drawing conclusions about the structure of the brain. The research, published in Current Biology, was conducted at the University of Freiburg and at the Research Institute of Molecular Pathology (IMP) in Vienna, Austria.
The newly developed method focuses on enhancers, DNA segments responsible for enhancing transcription of RNA at specific locations and developmental times in an organism. The research started with a database of three-dimensional images showing individual enhancer activity. The team used an automatic pattern finding algorithm to identify genetic activity patterns shared across the images. They noticed that, in some cases, these patterns seemed to correspond with specific brain regions. To demonstrate the functionality of their method, the biologists began by applying it to regions of the fruit fly brain whose anatomy is already well known – namely, those responsible for the sense of smell. The activity patterns of the enhancers traced the already familiar anatomy of these regions.
Then the biologists used the new method to study brain regions responsible for vision. These experiments led to new insights into the anatomy of these areas: In addition to eleven already known regions, the activity patterns of the enhancers revealed 14 new regions, each of which presumably serves a different function for the fruit fly’s sense of sight. The researchers now aim to conduct further studies to determine which regions are responsible for which functions.
Andrew Straw has served since January 2016 as professor of behavioral neurobiology and animal physiology at the University of Freiburg’s Faculty of Biology and is a member of the Bernstein Center Freiburg (BCF). Before their move to Freiburg, he and his research assistants Karin Panser and Dr. Laszlo Tirian worked at the Research Institute of Molecular Pathology in Vienna in collaboration with Dr. Florian Schulze, Virtual Reality and Visualization Research Center GmbH (VRVis). The goal of Straw’s research is to achieve a better understanding of the structure and function of the brain. He hopes this basic research will ultimately help in the design of therapies for patients suffering from neurological diseases affecting specific regions of the brain.
Results and visualizations: https://strawlab.org/braincode
Most cones don’t really see color
We see color because of specialized light-sensing cells in our eyes called cones. One type, L-cones, sees the reds of strawberries and fire trucks; M-cones detect green leaves, and S-cones let us know the sky is blue. But vision scientists have now discovered that not all cones sense color (see video). The finding was made possible because, for the first time, scientists were able to look at individual photo-sensing cells.

This Week in Chemistry: The chemical cause of Russia’s red river, a possible alternative to plastic microbeads in soaps, and more! Links to articles and studies here: https://goo.gl/wPQ5ym






Dancing Flames - Aluminium’s reactivity
aluminium’s protective oxide layer can make it difficult to see its true reactivity in the context of metals reacting with aqueous solutions. (Do not try to recreate experiment without the presence of a trained professional)
Procedure:
Dissolve copper(II) chloride in the hydrochloric acid and set the solution aside. Take a piece of aluminium foil approximately the width of the base of the conical flask and approximately 20 cm long. Roll it loosely just enough to be able to fit through the neck of the flask – use a splint or spatula to gently push it home so it lies on its side on the base of the flask. Have a source of ignition nearby with some splints. Pour the solution inside the flask and swirl the flask gently to get the reaction going.
After a few seconds, the mixture will begin to react vigorously and produce hydrogen gas. Hold the lit splint by the opening of the flask and the gas will ignite. If you have timed it right, the flame will sink back into the flask and dance inside above the reaction with an eerie green color from the copper.
Reaction:
2Al(s) + 3CuCl2(aq) → 2AlCl3(aq) + 3Cu(s)
2Al(s) + 6HCl(aq) → 2AlCl3(aq) + 3H2(g)
The primary objective here is to show how reactive aluminium is. The aluminium oxide layer protects the metal beneath from further reaction with air, water or acid. But chloride ions can ligate aluminium ions at the metal–oxide interface and break down the protective layer, allowing the reaction to proceed. -rsc
Giffed by: rudescience From: This video



This illustrator’s new book is a clever introduction to women scientists through history.
During a dinnertime discussion two years ago, illustrator Rachel Ignotofsky and her friend started chewing on the subject of what’s become a meaty conversation in America: women’s representation in STEM fields.
Ignotofsky, who lives in Kansas City, Missouri, lamented that kids don’t seem to hear much about women scientists. “I just kept saying over and over and over again that we’re not taught the stories of these women when we’re in school,” she recalls. Eventually, it dawned on her: “I was saying it enough that I was like, you know, I’m just talking a lot about this; I should draw some of the women in science that I feel really excited about.”
Learn more here.
[Reprinted with permission from Women in Science Copyright ©2016 by Rachel Ignotofsky. Published by Ten Speed Press, an imprint of Penguin Random House LLC.]

The Portuguese man o’ war delivers a powerful sting to its prey—and sometimes to people—through venom-filled structures on its tentacles. It is not a jellyfish, but rather a colony of different types of zooids (small animals). Jean Louis Coutant engraved the plate for this illustration.

Plantibodies and Plant-Derived Edible Vaccines
Throughout history, humans have used plants in the treatment of disease. This includes more traditional methods involving direct consumption with minimal preparation involved and the extraction of compounds for use in modern pharmaceuticals. One of the more recent methods of using plants in medicine involves the synthesis and application of plantibodies and plant produced antigens. These are recombinant antibodies and antigens respectively, which have been produced by a genetically modified plant (1, 2).
Antibodies are a diverse set of proteins which serve the purpose of aiding the body in eliminating foreign pathogens. They are secreted by effector B lymphocytes which are a type of white blood cell that circulate throughout the body. An antigen is a molecule or a component of a molecule, such as a protein or carbohydrate, which can stimulate an immune response. The human body is capable of producing around 1012 different types of antibodies, each of which can bind to a specific antigen or a small group of related motifs (3). When an antibody encounters the antigen of a foreign pathogen to which it has high affinity, it binds to it which can disable it or alert the immune system for its destruction (4).

Figure 1: Each type of antibody has the ability to bind to a specific antigen or group of antigens with high affinity.
Plants do not normally produce antibodies and thus must be genetically modified to produce plantibodies as well as foreign protein antigens. Plantibodies produced in this manner function the same way as the antibodies native to the human body (1). The main ways to do this are to stably integrate foreign DNA into a host cell and place it into a plant embryo resulting in a permanent change of the nuclear genome, or to induce transient gene expression of the specified protein (5). In both cases, the genetic material introduced to the plant codes for the protein of choice. Several of the methods used to induce permanent transgene expression include agrobacterium-mediated transformation, particle bombardment using a gene gun, or the transformation of organelles such as chloroplasts. Transient transgene expression can be done using plant viruses as viral vectors or agroinfiltration (2). Once the genetic material has been inserted, the specified protein is produced via the plant endomembrane and secretory systems, after which it can be recovered through purification of the plant tissue to be used for injection (1). The production of these proteins can also be directed to specific organs of the plant such as the seeds using targeting signals (2). Stable integration techniques are generally used for more large scale production and when the gene in question has a high level of expression, while transient techniques are used to produce a greater yield in the short term (5).

Figure 2: A gene gun being used to introduce genetic material into the leaves of a plant.
Now how can plantibodies and plant produced antigens help us as humans? The primary purpose of producing plantibodies is for the treatment of disease via immunotherapy. Immunotherapy is a method of treatment in which one’s immune response to a particular disease is enhanced. Specific plantibodies can be produced in order to target a particular disease and then be applied to patients via injection as a means of treatment (6). Doing so provides a boost to the number of antibodies against the targeted disease in the patient’s body which helps to enhance their immune system response against it. An example of this is CaroRx, the first clinically tested plantibody which has the ability to bind to Streptococcus mutans. CaroRx has been shown to be effective in the treatment of tooth decay caused by this species of bacteria (1). More recently, a plantibody known as ZMapp has shown potential in the treatment of Ebola. A study by Qiu et al showed that when administered up to 5 days after the onset of the disease, 100% of rhesus macaques that were administered the drug were shown to have recovered from its effects while all of the control group animals perished as a result of the disease (7). In addition, it has been experimentally administered to some humans who later recovered from the disease, although its role in their recovery was not fully ascertained (8).
Plant produced antigens on the other hand can be used to produce oral vaccines (9). Vaccines are typically biological mixtures containing a weakened pathogen and its antigens. Injection of this results in priming of the body’s adaptive immune system against the particular pathogen so that it can more easily recognize and respond to the threat in the future (4). By producing the antigens of targeted pathogens in plants through transgenic expression, edible vaccines can be created if the plant used is safe to eat. Tobacco, potato, and tomato plants have typically been used in past attempts to create them, showing success in both animal studies and a number of human trials. The advantages of using an oral vaccine include ease of administration and lower costs since specialised personel are not required for administration (9). In addition, oral vaccines are more effective in providing immunity against pathogens at mucosal surfaces as they can be directly applied to the gastrointestinal tract (1). The primary issue with the usage of oral vaccines is that protein antigens must avoid degradation in the stomach and intestines before they can reach the targeted sites in the body. Several solutions to this dilemma include using other biological structures such as liposomes and proteasomes as a means of delivery. This helps to prevent the proteins from being degraded by digestive enzymes and the acidic environment of the stomach before they can reach their destination (1, 9).

Figure 3: An overview of one method of producing an edible vaccine using a potato plant. A gene coding for the protein of a human pathogen is used in agrobacterium-mediated transformation to produce a transgenic potato plant. The potatoes from this plant can then serve as an edible vaccine against pathogen from which the protein originated.
There are a number of advantages to using these plant based pharmaceuticals. First of all, they can be produced on a large scale at a relatively low cost through agriculture and are convenient for long-term storage due to the resiliency and size of plant seeds (5). There is also a low risk of contamination by mammalian viruses, blood borne pathogens, and oncogenes which can remove the need for expensive removal steps (1). In addition, purification steps can be skipped if the plants used are edible and ethical problems that come with animal production can be avoided (5). The disadvantages include the potential for allergic reactions to plant antigens and contamination by pesticides and herbicides. There is also the possibility of outcrossing of transgenic pollen to weeds or related crops which would lead to non-target crops also expressing the pharmaceutical.This could lead to public concern along with the potential that other species which ingest these plants may be negatively affected (9). While plantibodies and plant produced antigens have not yet been extensively tested in clinical trials, going forward they represent a new treatment option with great promise.
References
1. Jain P, Pandey P, Jain D, Dwivedi P. Plantibody: An overview. Asian journal of Pharmacy and Life Science. 2011 Jan;1(1):87-94.
2. Stoger E, Sack M, Fischer R, Christou P. Plantibodies: applications, advantages and bottlenecks. Current Opinion in Biotechnology. 2002 Apr 1;13(2):161-166.
3. Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. 4th Edition. New York: Garland Science; 2002.
4. Parham P. The immune system. 4th Edition. New York: Garland Science; 2014.
5. Ferrante E, Simpson D. A review of the progression of transgenic plants used to produce plantibodies for human usage. J. Young Invest. 2001;4:1-0.
6. Smith MD. Antibody production in plants. Biotechnology advances. 1996 Dec 31;14(3):267-81.
7. Qiu X, Wong G, Audet J, Bello A, Fernando L, Alimonti JB, Fausther-Bovendo H, Wei H, Aviles J, Hiatt E, Johnson A. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature. 2014 Aug 29.
8. Sneed A. Know the Jargon. Scientific american. 2014 Dec 1;311(6):24-24.
9. Daniell H, Streatfield SJ, Wycoff K. Medical molecular farming: production of antibodies, biopharmaceuticals and edible vaccines in plants. Trends in plant science. 2001 May 1;6(5):219-26.

Anti-microbial Peptides: Proteins that Pack a Punch
Antimicrobial resistance is a growing concern and it is currently estimated that approximately 2 million people are infected annually with serious infections that show antibiotic resistance to some degree. This contributes to the mortality of 23, 000 people with many more suffering severe complications as a direct result of antibiotic resistant infections. The economic burden on the US is thought to exceed $20 billion simply on health care bills alone, and a further $35 billion due to a societal loss in work based productivity (1).
The spread of antibiotic resistance is now widely believed to be a direct result of the anthropogenic release of antibiotics into the biosphere. We are now faced with the dilemma of how to treat these infections. In previous articles, I’ve talked largely about bacteriophages and how they are one possible solution to this complex problem. This article will introduce you to another class of antimicrobial agents, aptly called antimicrobial peptides (AMPs).
What are Antimicrobial Peptides?
Proteins are found ubiquitously throughout all cellular life and are like the mechanical parts of a car, helping your cells carry out a vast array of functions every single day. Peptides are small proteins that contain two or more amino acids joined by peptide bonds. Anyone who is familiar with biochemistry will be aware of the sheer diversity found amongst these versatile molecules. Needless to say, it should not be surprising that there are a large class of proteins involved in offensive cellular warfare. They are found widely in all domains of life and have evolved to give a cell a competitive advantage over its nastier neighbours.
Without getting too bogged down with the biochemistry, AMPs are characterised by their overall properties. AMPs that share common structural features will also have a similar function when targeting a cell. The diversity amongst these proteins can be seen in Figure 1, which shows some examples from the four classes of AMPs. The class I AMPs, the lantibiotics for example, all contain similar motifs which assign them a similar job. AMPs can range from anywhere between 6 to >59 amino acids, but are generally considered to be small proteins (2). They generally have a rather amphipathic nature and feature both positive and negative charges.
These peptides may have a number of rare (Figure 1), modified amino acids. The lanthionines are a class of AMP that contain lanthionine rings made from dehydrated serine and threonine residues connected by thioether cross-links. This happens after the protein leaves the ribosome and gives the protein some very unique properties which will be explained later in the article (3).
Figure 1. The four classes of AMPs, showing common examples in each class. Rare, modified amino acids are indicated by coloured circles with the three letter codes indicating the name of the residue. Thioether cross-links are indicated by an S coordinated by two black lines (3).
Implications for the Pharmaceutical industry
Our antibiotic pipeline is drying up (Figure 2), with few new drugs being approved by the Food and Drug Administration. Identifying novel antibiotics is a tedious process that requires a lot of time and effort from drug companies, which they are not willing to do. The reason for this boils down to economic reasons, as antibiotics are just not worth the investment. Unlike other drugs such as statins, antibiotics are only used for short periods of time by a patient. One course of treatment therefore doesn’t return a massive profit for the company. The second issue antibiotics face is that resistance to them occurs rapidly after they are put into circulation, so the company is not likely to get much use out of the drug. Therefore we need to find a new source for our antimicrobials. This is where the AMPs come in.
Currently, nearly 900 AMPs have been identified and characterised with many more undiscovered (2). They are an untapped source of drug discovery and they exhibit numerous benefits over their antibiotic cousins. As they are proteins, they have a genetic origin, which could provide an amenable platform for further development through random mutagenesis. This could produce a vast library of antimicrobial compounds (4,5), drastically improving our options for therapy.
Figure 2. Graph showing the steady decline in antibiotic development from 1980 to 2012 (1)
Nisin; not so nice if you’re a bacterial cell
AMPs were discovered in the 1930s although their use in the health industry has been fairly limited, resulting from the sheer difficulty and cost of manufacturing and purifying proteins on a large scale. The bacterially produced lantibiotics are by far the most well studied AMPs and have the most potential for the pharmaceutical industry. Nisin (E234) is the most well studied lantibiotic (Refer to Figure 1, Class I) and is produced by the bacterium Lactococcus lactis (6).
It shows broad spectrum activity on a large number of Gram-positive bacteria including other lactic acid bacteria, which has made it a coveted preservative in food processing. Currently it is added to cheeses, meats and beverages to extend shelf life and prevent the growth of spoilage organisms including spore forming bacteria such as Clostridium botulinum (6). The lantibiotics have also proven their capabilities for treating the clinically relevant pathogens methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococci (7). They are also seen to have similar levels of activity as antibiotics and express low levels of toxicity to mammalian cells. Nisin exhibits poor oral availability making it more appealing as a topical agent or for intravenous application but there are also intentions to use it as a sterilising agent for catheters and medical equipment to help reduce the risk of infection (3).
So could lantibiotics like nisin be a good solution to antimicrobial resistance? Well more compelling evidence for nisin is that resistance has not been thoroughly documented. Nisin has been applied in sub-therapeutic concentrations in the food industry since the 1960s but still mostly retains its bactericidal ability. Resistance has been achieved artificially in the lab (discussed later in the article), but due to the mechanism of some lantibiotics including nisin, resistance is thought to be unlikely (6).
The mechanism behind nisin’s potency
Unlike animal cells, generally bacterial membranes have an overall negative charge and lack cholesterol (8). Nisin contains a high proportion of the positively charged (basic) amino acids lysine and arginine. These positive charges allow the protein to interact with the negative charges commonly associated with bacterial cell membranes (2). Nisin is good at aligning against Gram-positive bacterial membranes, where they multimerise to form short-lived pores (Figure 3). Hydrophobic regions help the protein to insert into the membrane and stabilise the pore (2), which allows the transport of ATP, ions and amino acids, eliminating the cellular membrane potential (9).
Nisin has a second trick up its sleeve. Its C-terminal, the portion of the protein containing the lanthionine ring motifs, allows it to latch onto the important membrane component lipid II (Figure 3). Lipid II is a precursor for peptidoglycan; the cell wall strengthening polymer found in both Gram-positive and Gram-negative bacteria. It is a common target for antibiotics including penicillin and vancomycin, which both target different stages of its synthesis. It helps to maintain the cell structure and prevents it from bursting under high osmotic pressure. When nisin binds to lipid II, it sequesters this molecule from the enzymes that catalyse its addition to growing peptidoglycan chains. Binding lipid II also helps to stabilise the transmembrane pores, further damaging the cell. As a result, not only is the cell wall weakened, but the cell loses its metabolic capabilities, through the loss of charged molecules.
The dual targeting system of nisin is thought to be the reason why resistance to nisin has not be well documented (10). The two processes are completely physiologically separate, and therefore to develop resistance, the bacteria would have to develop two unrelated mutations to counteract the effects of nisin.
Figure 3. Diagram showing the mechanism of several lantibiotics including nisin. AMPs are represented by lines made with clear circles. Phospholipids represented by green circles with tails. Lipid II is represented by orange hexagons (3).
What do we know about resistance towards nisin?
There are several proposed means by which an organism can be resistance to a toxin. Firstly, an organism may have innate immunity to a toxin simply because of its physiology. We see this largely in the Gram-negative bacteria towards nisin. The lipopolysaccharide (LPS) layer found on the outside of their cell wall provides protection against nisin and it has been shown that the oligosaccharides found within the core region of this structure greatly improve protection against nisin. It is believed that this is because metal ions are sequestered within this layer, adding additional positive charges to the site. Such charges would help to prevent nisin from aligning with the cell membrane (11). Removing these metal ions by sequestering them sensitises Gram-negative bacteria to nisin.
Emergent resistance is the type of resistance that should concern us the most, as it is the reason why we are now faced with the problem of antimicrobial resistance. It involves the acquisition of mutations or DNA that help confer tolerance to stress resulting from the action of a toxin (12). Although currently only produced in the laboratory, experiments carried out on the tolerance of clinically relevant bacteria towards nisin are crucial in highlighting the future of implementing an antimicrobial.
Resistance mechanisms have been documented in several bacteria including the causative agent of listeriosis, Listeria monocytogenes. Although not fully understood, changes in membrane composition have been attributed for the decreased susceptibility in resistant strains. In resistant strains, the bacterial membrane is composed of less negatively charged phospholipids. Similarly to sequestering metal ions near the membrane, this alters the overall net charge, helping to repel nisin.
The number of long chain fatty acids within its membrane is increased helping to reducing fluidity. This is believed to play a role in preventing nisin from inserting itself into the membrane. Studies show that nisin resistant strains were also less susceptible to cell wall acting components such as lysozyme and cell wall acting antibiotics. They did not identify the phenotypic change that gave additional protection, but this does indicate that a number of defence mechanisms are involved in defending cells against environmental stress from nisin (13).
Conclusion:
So could AMPs like nisin possibly serve as a replacement to our current armamentarium of antibiotics? AMPs are a largely untapped source of antimicrobials with many more still to be identified. AMPs may therefore serve as a new source of antimicrobials to help relieve the stress exerted on microorganisms by antibiotics. We have seen that nisin is an effective antimicrobial against a wide range of Gram-positive bacteria including spore forming bacteria. The dual-action of nisin challenges bacterial cells making it difficult for them to develop resistance. However, lab-based experiments have shown that it is possible to generate resistant strains showing the tenacity of bacteria to adapt to such potent environmental stresses. To learn from our previous mistakes with antibiotics, more responsible practices would need to be applied. Using combination therapy or rotating drug usage, as done with pesticides, could help further prevent resistance. Where they are likely to be applied in high concentration (in medical settings and agriculture), combination therapies should be used to further reduce the likelihood of resistance.
1. CDC. Antibiotic resistance threats. US Dep Healh Hum Serv. 2013;22–50.
2. Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol [Internet]. 2005;3(3):238–50. Available from: http://www.nature.com/doifinder/10.1038/nrmicro1098
3. Dischinger J, Basi Chipalu S, Bierbaum G. Lantibiotics: Promising candidates for future applications in health care. Int J Med Microbiol [Internet]. Elsevier GmbH.; 2014;304(1):51–62. Available from: http://dx.doi.org/10.1016/j.ijmm.2013.09.003
4. Field D, Begley M, O’Connor PM, Daly KM, Hugenholtz F, Cotter PD, et al. Bioengineered Nisin A Derivatives with Enhanced Activity against Both Gram Positive and Gram Negative Pathogens. PLoS One. 2012;7(10).
5. Hilpert K, Volkmer-Engert R, Walter T, Hancock REW. High-throughput generation of small antibacterial peptides with improved activity. Nat Biotechnol. 2005;23(8):1008–12.
6. van Heel AJ, Montalban-Lopez M, Kuipers OP. Evaluating the feasibility of lantibiotics as an alternative therapy against bacterial infections in humans. Expert Opin Drug Metab Toxicol. 2011;7(6):675–80.
7. Barbosa J, Caetano T, Mendo S. Class I and Class II Lanthipeptides Produced by Bacillus spp. J Nat Prod [Internet]. 2015;151008121848005. Available from: http://pubsdc3.acs.org/doi/10.1021/np500424y
8. Neumann A, Berends ETM, Nerlich A, Molhoek EM, Gallo RL, Meerloo T, et al. The antimicrobial peptide LL-37 facilitates the formation of neutrophil extracellular traps. Biochem J [Internet]. 2014 Nov 15 [cited 2014 Oct 28];464(1):3–11. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25181554
9. Kordel M, Schuller F, Sahl HG. Interaction of the pore forming-peptide antibiotics Pep 5, nisin and subtilin with non-energized liposomes. FEBS Lett. 1989;244(1):99–102.
10. Islam MR, Nagao J, Zendo T, Sonomoto K. Antimicrobial mechanism of lantibiotics. Biochem Soc Trans [Internet]. 2012;40(6):1528–33. Available from: http://www.biochemsoctrans.org/bst/040/bst0401528.htm
11. Stevens K a., Sheldon BW, Klapes N a., Klaenhammer TR. Nisin treatment for inactivation of Salmonella species and other gram- negative bacteria. Appl Environ Microbiol. 1991;57(12):3613–5.
12. Kaur G, Malik RK, Mishra SK, Singh TP, Bhardwaj A, Singroha G, et al. Nisin and class IIa bacteriocin resistance among Listeria and other Foodborne pathogens and spoilage bacteria. Microb Drug Resist. 2011;17(2).
13. Crandall AD, Montville TJ. Nisin resistance in Listeria monocytogenes ATCC 700302 is a complex phenotype. Appl Environ Microbiol. 1998
The gut bacteria inside 1000-year-old mummies from the Inca Empire are resistant to most of today’s antibiotics, even though we only discovered these drugs within the last 100 years.
“At first we were very surprised,” Tasha Santiago-Rodriguez of California Polytechnic State University in San Louis Opisbo, told the Annual Meeting of the American Society for Microbiology last month.
Her team studied the DNA within the guts of three Incan mummies dating back to between the 10th and 14thcenturies and six mummified people from Italy, from between the 15th and 18th centuries. They found an array of genes that have the potential to resist almost all modern antibiotics, including penicillin, vancomycin and tetracycline.
These ancient genes were largely in microbes whose resistance is problematic today, including Enteroccocus bacteria that can infect wounds and cause urinary tract infections. But they found that many other species, including some harmless ones, carried some of these resistant genes too.
Enterococcus enigma
“When you think about it, almost all these antibiotics are naturally produced, so it makes sense to find antibiotic genes as well,” says Santiago-Rodriguez.
Their finding shows that genes that can confer resistance to antibiotics were relatively widespread hundreds of years before Alexander Fleming discovered penicillin in 1928. “It’s ridiculous to think evolution of antibiotic resistance began when penicillin was discovered,” said team-member Raul Cano, also at California Polytechnic State University, at the meeting while discussing the findings. “It’s been going on for 2 billion years.”
These genes existed long before antibiotics became common, but it is our overuse of these drugs in both people and livestock that caused the superbug resistance to explode worldwide, said Cano.
“This is exciting data,” says Adam Roberts, who studies antibiotic resistance genes at University College London. While it is already well known that antibiotic resistance occurred naturally before people started using antibiotics, this study shows that resistance genes were already within the human gut long before we started using these drugs, he says.
“It begs the question of what was selecting for these genes at this time? Was it the natural production of antibiotics by other bacteria, or were there other, as yet unknown forces at play?” asks Roberts.
Are colour-changing octopuses really colourblind?
Cephalopods, including octopuses and squid, have some of the most incredible colour-changing abilities in nature.
They can almost instantly blend in with their surroundings to evade predators or lay in wait, and put on colourful displays to attract mates or dazzle potential prey.
This is impressive enough on its own, but becomes even more amazing when you discover these creatures are in fact colourblind – they only have one type of light receptor in their eyes, meaning they can only see in black and white.
So how do they know what colours to change to at all?
This has puzzled biologists for decades but a father/son team of scientists from the University of California, Berkeley, and Harvard University think the unusual shape of their pupils holds the key, and they can see colour after all.
Cephalopods have wide U-shaped or dumbbell-shaped pupils, which allow light into the lens from many directions.

When light enters the pupils in human eyes it gets focused on one spot, cutting down on blur from the light being split into its constituent colours.
The scientists believe cephalopod eyes work the opposite way – the wide pupils split the light up and then individual colours can be focused on the retina by changing the depth of the eyeball and moving the pupil around.
The price for this is blurry vision, but it does mean they could make out colours in a unique way to any other animals.
Processing colour this way is more computationally intensive than other types of colour vision and likely requires a lot of brainpower, which might explain in part why cephalopods are the most intelligent invertebrates on Earth.

Read the paper
Images: Roy Caldwell, Klaus Stiefel, Alexander Stubbs
It is imperfection - not perfection - that is the end result of the program written into that formidably complex engine that is the human brain, and of the influences exerted upon us by the environment and whoever takes care of us during the long years of physical, psychological and intellectual development.
Rita Levi-Montalcini

Image Credit: Hammersmith Hospital in London
(via neuromorphogenesis)

Manufacturing Dopamine in the Brain with Gene Therapy
Parkinson’s patients who take the drug levodopa, or L-Dopa, are inevitably disappointed. At first, during a “honeymoon” period, their symptoms (which include tremors and balance problems) are brought under control. But over time the drug becomes less effective. They may also need ultrahigh doses, and some start spending hours a day in a state of near-frozen paralysis.
A biotech company called Voyager Therapeutics now thinks it can extend the effects of L-Dopa by using a surprising approach: gene therapy. The company, based in Cambridge, Massachusetts, is testing the idea in Parkinson’s patients who’ve agreed to undergo brain surgery and an injection of new DNA.
Parkinson’s occurs when dopamine-making neurons in the brain start dying, causing movement symptoms that afflicted boxing champ Muhammad Ali and actor Michael J. Fox, whose charitable foundation has helped pay for the development of Voyager’s experimental treatment.
The cause of Parkinson’s isn’t well understood, but the reason the drug wears off is. It’s because the brain also starts losing an enzyme known as aromatic L-amino acid decarboxylase, or AADC, that is needed to convert L-Dopa into dopamine.
Voyager’s strategy, which it has begun trying on patients in a small study, is to inject viruses carrying the gene for AADC into the brain, an approach it thinks can “turn back the clock” so that L-Dopa starts working again in advanced Parkinson’s patients as it did in their honeymoon periods.
Videos of patients before and after taking L-Dopa make it obvious why they’d want the drug to work at a lower dose. In the ‘off’ state, people move in slow motion. Touching one’s nose takes an effort. In an ‘on’ state, when the drug is working, they’re shaky, but not nearly so severely disabled.
“They do well at first but then respond very erratically to L-Dopa,” says Krystof Bankiewicz, the University of California scientist who came up with the gene-therapy plan and is a cofounder of Voyager. “This trial is to restore the enzyme and allow them to be awakened, or ‘on,’ for a longer period of time.”
Voyager was formed in 2013 and later went public, raising about $86 million. The company is part of a wave of biotechs that have been able to raise money for gene therapy, a technology that is starting to pay off: after three decades of research, a few products are reaching the market.
Unlike conventional drug studies, those involving gene therapy often come with very high expectations that the treatment will work. That’s because it corrects DNA errors for which the exact biological consequences are known. Genzyme, a unit of the European drug manufacturer Sanofi, paid Voyager $65 million and promised hundreds of millions more in order to sell any treatments it develops in Europe and Asia.
“We’re working with 60 years of dopamine pharmacology,” says Steven Paul, Voyager’s CEO, and formerly an executive at the drug giant Eli Lilly. “If we can get the gene to the right tissue at the right time, it would be surprising if it didn’t work.”
But those are big ifs. In fact, the concept for the Parkinson’s gene therapy dates to 1986, when Bankiewicz first determined that too little AADC was the reason L-Dopa stops working. He thought gene therapy might be a way to fix that, but it wasn’t until 20 years later that he was able to test the idea in 10 patients, in a study run by UCSF.
In that trial, Bankiewicz says, the gene delivery wasn’t as successful as anticipated. Not enough brain cells were updated with the new genetic information, which is shuttled into them by viruses injected into the brain. Patients seemed to improve, but not by much.
Even though the treatment didn’t work as planned, that early study highlighted one edge Voyager’s approach has over others. It is possible to tag AADC with a marker chemical, so doctors can actually see it working inside patients’ brains. In fact, ongoing production of the dopamine-making enzyme is still visible in the brains of the UCSF patients several years later.

It is possible to tag AADC with a marker chemical, so doctors can actually see it working inside patients’ brains. Image Source: MIT Technology Review.
In some past studies of gene therapy, by contrast, doctors had to wait until patients died to find out whether the treatment had been delivered correctly. “This is a one-and-done treatment,” says Paul. “And anatomically, it tells us if we got it in the right place.”
A new trial under way, this one being carried out by Voyager, is designed to get much higher levels of DNA into patients’ brains in hopes of achieving better results. To do that, Bankiewicz developed a system to inject the gene-laden viral particles through pressurized tubes while a patient lies inside an MRI scanner. That way, the surgeon can see the putamen, the brain region where the DNA is meant to end up, and make sure it’s covered by the treatment.
There are other gene therapies for Parkinson’s disease planned or in testing. A trial developed at the National Institutes of Health seeks to add a growth factor and regenerate cells. A European company, Oxford BioMedica, is trying to replace dopamine.
Altogether, as of this year, there were 48 clinical trials under way of gene or cell replacement in the brain and nervous system, according to the Alliance for Regenerative Medicine, a trade group. The nervous system is the fourth most common target for this style of experimental treatment, after cancer, heart disease, and infections.
Voyager’s staff is enthusiastic about a study participant they call “patient number 6,” whom they’ve been tracking for several months—ever since he got the treatment. Before the gene therapy, he was on a high dose of L-Dopa but still spent six hours a day in an “off” state. Now he’s off only two hours a day and takes less of the drug.
That patient got the highest dose of DNA yet, covering the largest brain area. That is part of what makes Voyager think higher doses should prove effective. “I believe that previous failure of gene-therapy trials in Parkinson’s was due to suboptimal delivery,” says Bankiewicz.
Image Credit: L.A. JOHNSON
Source: MIT Technology Review (by Antonio Regalado)

For those poorly informed (educated) who insist that vaccines are just the same as catching the illness…. This is just one example of why that is not true.
Breakthrough for vaccine research: Mucosa forms special immunological memory
If a vaccine is to protect the intestines and other mucous membranes in the body, it also needs to be given through the mucosa, for example as a nasal spray or a liquid that is drunk. The mucosa forms a unique immunological antibody memory that does not occur if the vaccine is given by injection. This has been shown by a new study from Sahlgrenska Academy published in Nature Communications.
Immunological memory is the secret to human protection against various diseases and the success of vaccines. It allows our immune system to quickly recognize and neutralize threats. “The largest part of the immune system is in our mucosa. Even so, we understand less about how immunological memory protects us there than we do about protection in the rest of the body. Some have even suggested that a typical immune memory function does not exist in the mucosa,” says Mats Bemark, associate professor of immunology at Sahlgrenska Academy, University of Gothenburg.
After extensive work, the research team at Sahlgrenska Academy can now show that this assumption is completely wrong.
Mats Bemark et al. Limited clonal relatedness between gut IgA plasma cells and memory B cells after oral immunization, Nature Communications (2016). DOI: 10.1038/ncomms12698

At last, we’ve seen what might be the primary building blocks of memories lighting up in the brains of mice.
We have cells in our brains – and so do rodents – that keep track of our location and the distances we’ve travelled. These neurons are also known to fire in sequence when a rat is resting, as if the animal is mentally retracing its path – a process that probably helps memories form, says Rosa Cossart at the Institut de Neurobiologie de la Méditerranée in Marseille, France.
But without a way of mapping the activity of a large number of these individual neurons, the pattern that these replaying neurons form in the brain has been unclear. Researchers have suspected for decades that the cells might fire together in small groups, but nobody could really look at them, says Cossart.
To get a look, Cossart and her team added a fluorescent protein to the neurons of four mice. This protein fluoresces the most when calcium ions flood into a cell – a sign that a neuron is actively firing. The team used this fluorescence to map neuron activity much more widely than previous techniques, using implanted electrodes, have been able to do.
Observing the activity of more than 1000 neurons per mouse, the team watched what happened when mice walked on a treadmill or stood still.
As expected, when the mice were running, the neurons that trace how far the animal has travelled fired in a sequential pattern, keeping track.
These same cells also lit up while the mice were resting, but in a strange pattern. As they reflected on their memories, the neurons fired in the same sequence as they had when the animals were running, but much faster. And rather than firing in turn individually, they fired together in sequential blocks that corresponded to particular fragments of a mouse’s run.
“We’ve been able to image the individual building-blocks of memory,” Cossart says, each one reflecting a chunk of the original episode that the mouse experienced.
Continue Reading.

Shocking New Role Found for the Immune System: Controlling Social Interactions
In a startling discovery that raises fundamental questions about human behavior, researchers at the University of Virginia School of Medicine have determined that the immune system directly affects – and even controls – creatures’ social behavior, such as their desire to interact with others.
So could immune system problems contribute to an inability to have normal social interactions? The answer appears to be yes, and that finding could have significant implications for neurological diseases such as autism-spectrum disorders and schizophrenia.
“The brain and the adaptive immune system were thought to be isolated from each other, and any immune activity in the brain was perceived as sign of a pathology. And now, not only are we showing that they are closely interacting, but some of our behavior traits might have evolved because of our immune response to pathogens,” explained Jonathan Kipnis, chair of UVA’s Department of Neuroscience. “It’s crazy, but maybe we are just multicellular battlefields for two ancient forces: pathogens and the immune system. Part of our personality may actually be dictated by the immune system.”
Evolutionary Forces at Work
It was only last year that Kipnis, the director of UVA’s Center for Brain Immunology and Glia, and his team discovered that meningeal vessels directly link the brain with the lymphatic system. That overturned decades of textbook teaching that the brain was “immune privileged,” lacking a direct connection to the immune system. The discovery opened the door for entirely new ways of thinking about how the brain and the immune system interact.

(Image caption: Normal brain activity, left, and a hyper-connected brain. Credit: Anita Impagliazzo, UVA Health System)
The follow-up finding is equally illuminating, shedding light on both the workings of the brain and on evolution itself. The relationship between people and pathogens, the researchers suggest, could have directly affected the development of our social behavior, allowing us to engage in the social interactions necessary for the survival of the species while developing ways for our immune systems to protect us from the diseases that accompany those interactions. Social behavior is, of course, in the interest of pathogens, as it allows them to spread.
The UVA researchers have shown that a specific immune molecule, interferon gamma, seems to be critical for social behavior and that a variety of creatures, such as flies, zebrafish, mice and rats, activate interferon gamma responses when they are social. Normally, this molecule is produced by the immune system in response to bacteria, viruses or parasites. Blocking the molecule in mice using genetic modification made regions of the brain hyperactive, causing the mice to become less social. Restoring the molecule restored the brain connectivity and behavior to normal. In a paper outlining their findings, the researchers note the immune molecule plays a “profound role in maintaining proper social function.”
“It’s extremely critical for an organism to be social for the survival of the species. It’s important for foraging, sexual reproduction, gathering, hunting,” said Anthony J. Filiano, Hartwell postdoctoral fellow in the Kipnis lab and lead author of the study. “So the hypothesis is that when organisms come together, you have a higher propensity to spread infection. So you need to be social, but [in doing so] you have a higher chance of spreading pathogens. The idea is that interferon gamma, in evolution, has been used as a more efficient way to both boost social behavior while boosting an anti-pathogen response.”
Understanding the Implications
The researchers note that a malfunctioning immune system may be responsible for “social deficits in numerous neurological and psychiatric disorders.” But exactly what this might mean for autism and other specific conditions requires further investigation. It is unlikely that any one molecule will be responsible for disease or the key to a cure. The researchers believe that the causes are likely to be much more complex. But the discovery that the immune system – and possibly germs, by extension – can control our interactions raises many exciting avenues for scientists to explore, both in terms of battling neurological disorders and understanding human behavior.
“Immune molecules are actually defining how the brain is functioning. So, what is the overall impact of the immune system on our brain development and function?” Kipnis said. “I think the philosophical aspects of this work are very interesting, but it also has potentially very important clinical implications.”
Findings Published
Kipnis and his team worked closely with UVA’s Department of Pharmacology and with Vladimir Litvak’s research group at the University of Massachusetts Medical School. Litvak’s team developed a computational approach to investigate the complex dialogue between immune signaling and brain function in health and disease.
“Using this approach we predicted a role for interferon gamma, an important cytokine secreted by T lymphocytes, in promoting social brain functions,” Litvak said. “Our findings contribute to a deeper understanding of social dysfunction in neurological disorders, such as autism and schizophrenia, and may open new avenues for therapeutic approaches.”
The findings have been published online by the prestigious journal Nature.
Using the Power of Space to Fight Cancer
From cancer research to DNA sequencing, the International Space Space is proving to be an ideal platform for medical research. But new techniques in fighting cancer are not confined to research on the space station. Increasingly, artificial intelligence is helping to “read” large datasets. And for the past 15 years, these big data techniques pioneered by our Jet Propulsion Laboratory have been revolutionizing biomedical research.
Microgravity Research on Space Station
On Earth, scientists have devised several laboratory methods to mimic normal cellular behavior, but none of them work exactly the way the body does. Beginning more than 40 years ago aboard Skylab and continuing today aboard the space station, we and our partners have conducted research in the microgravity of space. In this environment, in vitro cells arrange themselves into three-dimensional groupings, or aggregates. These aggregates more closely resemble what actually occurs in the human body. Cells in microgravity also tend to clump together more easily, and they experience reduced fluid shear stress – a type of turbulence that can affect their behavior. The development of 3D structure and enhanced cell differentiation seen in microgravity may help scientists study cell behavior and cancer development in models that behave more like tissues in the human body.

In addition, using the distinctive microgravity environment aboard the station, researchers are making further advancements in cancer therapy. The process of microencapsulation was investigated aboard the space station in an effort to improve the Earth-based technology. Microencapsulation is a technique that creates tiny, liquid-filled, biodegradable micro-balloons that can serve as delivery systems for various compounds, including specific combinations of concentrated anti-tumor drugs. For decades, scientists and clinicians have looked for the best ways to deliver these micro-balloons, or microcapsules, directly to specific treatment sites within a cancer patient, a process that has the potential to revolutionize cancer treatment.

A team of scientists at Johnson Space Center used the station as a tool to advance an Earth-based microencapsulation system, known as the Microencapsulation Electrostatic Processing System-II (MEPS-II), as a way to make more effective microcapsules. The team leveraged fluid behavior in microgravity to develop a new technique for making these microcapsules that would be more effective on Earth. In space, microgravity brought together two liquids incapable of mixing on Earth (80 percent water and 20 percent oil) in such a way that spontaneously caused liquid-filled microcapsules to form as spherical, tiny, liquid-filled bubbles surrounded by a thin, semipermeable, outer membrane. After studying these microcapsules on Earth, the team was able to develop a system to make more of the space-like microcapsules on Earth and are now performing activities leading to FDA approval for use in cancer treatment.

In addition, the ISS National Laboratory managed by the Center for the Advancement of Science in Space (CASIS) has also sponsored cancer-related investigations. An example of that is an investigation conducted by the commercial company Eli Lilly that seeks to crystallize a human membrane protein involved in several types of cancer together with a compound that could serve as a drug to treat those cancers.
“So many things change in 3-D, it’s mind-blowing – when you look at the function of the cell, how they present their proteins, how they activate genes, how they interact with other cells,” said Jeanne Becker, Ph.D., a cell biologist at Nano3D Biosciences in Houston and principal investigator for a study called Cellular Biotechnology Operations Support Systems: Evaluation of Ovarian Tumor Cell Growth and Gene Expression, also known as the CBOSS-1-Ovarian study. “The variable that you are most looking at here is gravity, and you can’t really take away gravity on Earth. You have to go where gravity is reduced.“
Crunching Big Data Using Space Knowledge

Our Jet Propulsion Laboratory often deals with measurements from a variety of sensors – say, cameras and mass spectrometers that are on our spacecraft. Both can be used to study a star, planet or similar target object. But it takes special software to recognize that readings from very different instruments relate to one another.
There’s a similar problem in cancer research, where readings from different biomedical tests or instruments require correlation with one another. For that to happen, data have to be standardized, and algorithms must be “taught” to know what they’re looking for.
Because space exploration and cancer research share a similar challenge in that they both must analyze large datasets to find meaning, JPL and the National Cancer Institute renewed their research partnership to continue developing methods in data science that originated in space exploration and are now supporting new cancer discoveries.
JPL’s methods are leading to the development of a single, searchable network of cancer data that researcher can work into techniques for the early diagnosis of cancer or cancer risk. In the time they’ve worked together, the two organizations’ efforts have led to the discovery of six new Food and Drug Administration-approved cancer biomarkers. These agency-approved biomarkers have been used in more than 1 million patient diagnostic tests worldwide.





