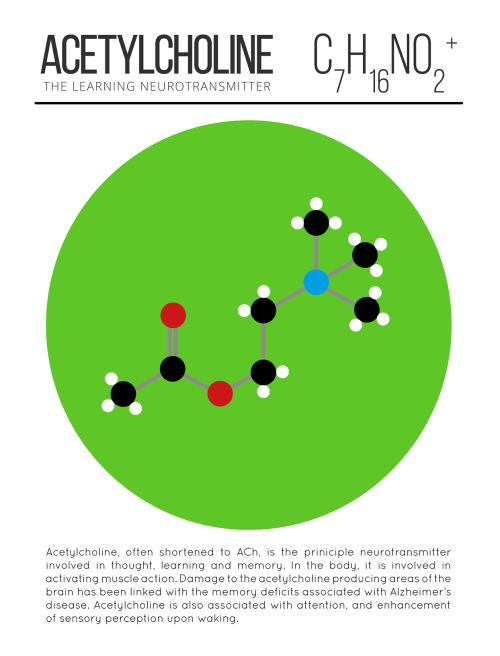
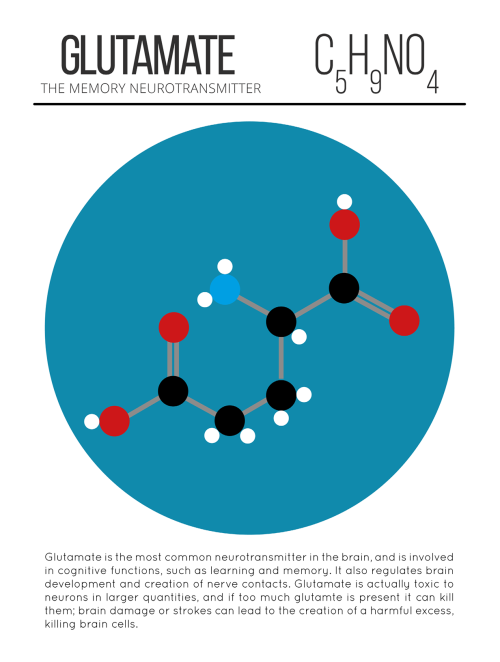
A pharmacist and a little science sideblog. "Knowledge belongs to humanity, and is the torch which illuminates the world." - Louis Pasteur
215 posts
Latest Posts by contradictiontonature - Page 7

(Image caption: Brandeis University professor Ricardo Godoy conducts the experiment in a village in the Bolivian rainforest. The participants were asked to rate the pleasantness of various sounds, and Godoy recorded their response. Credit: Alan Schultz)
Why we like the music we do
In Western styles of music, from classical to pop, some combinations of notes are generally considered more pleasant than others. To most of our ears, a chord of C and G, for example, sounds much more agreeable than the grating combination of C and F# (which has historically been known as the “devil in music”).
For decades, neuroscientists have pondered whether this preference is somehow hardwired into our brains. A new study from MIT and Brandeis University suggests that the answer is no.
In a study of more than 100 people belonging to a remote Amazonian tribe with little or no exposure to Western music, the researchers found that dissonant chords such as the combination of C and F# were rated just as likeable as “consonant” chords, which feature simple integer ratios between the acoustical frequencies of the two notes.
“This study suggests that preferences for consonance over dissonance depend on exposure to Western musical culture, and that the preference is not innate,” says Josh McDermott, the Frederick A. and Carole J. Middleton Assistant Professor of Neuroscience in the Department of Brain and Cognitive Sciences at MIT.
McDermott and Ricardo Godoy, a professor at Brandeis University, led the study, which appeared in Nature on July 13. Alan Schultz, an assistant professor of medical anthropology at Baylor University, and Eduardo Undurraga, a senior research associate at Brandeis’ Heller School for Social Policy and Management, are also authors of the paper.
Consonance and dissonance
For centuries, some scientists have hypothesized that the brain is wired to respond favorably to consonant chords such as the fifth (so-called because one of the notes is five notes higher than the other). Musicians in societies dating at least as far back as the ancient Greeks noticed that in the fifth and other consonant chords, the ratio of frequencies of the two notes is usually based on integers — in the case of the fifth, a ratio of 3:2. The combination of C and G is often called “the perfect fifth.”
Others believe that these preferences are culturally determined, as a result of exposure to music featuring consonant chords. This debate has been difficult to resolve, in large part because nowadays there are very few people in the world who are not familiar with Western music and its consonant chords.
“It’s pretty hard to find people who don’t have a lot of exposure to Western pop music due to its diffusion around the world,” McDermott says. “Most people hear a lot of Western music, and Western music has a lot of consonant chords in it. It’s thus been hard to rule out the possibility that we like consonance because that’s what we’re used to, but also hard to provide a definitive test.”
In 2010, Godoy, an anthropologist who has been studying an Amazonian tribe known as the Tsimane for many years, asked McDermott to collaborate on a study of how the Tsimane respond to music. Most of the Tsimane, a farming and foraging society of about 12,000 people, have very limited exposure to Western music.
“They vary a lot in how close they live to towns and urban centers,” Godoy says. “Among the folks who live very far, several days away, they don’t have too much contact with Western music.”
The Tsimane’s own music features both singing and instrumental performance, but usually by only one person at a time.
Dramatic differences
The researchers did two sets of studies, one in 2011 and one in 2015. In each study, they asked participants to rate how much they liked dissonant and consonant chords. The researchers also performed experiments to make sure that the participants could tell the difference between dissonant and consonant sounds, and found that they could.
The team performed the same tests with a group of Spanish-speaking Bolivians who live in a small town near the Tsimane, and residents of the Bolivian capital, La Paz. They also tested groups of American musicians and nonmusicians.
“What we found is the preference for consonance over dissonance varies dramatically across those five groups,” McDermott says. “In the Tsimane it’s undetectable, and in the two groups in Bolivia, there’s a statistically significant but small preference. In the American groups it’s quite a bit larger, and it’s bigger in the musicians than in the nonmusicians.”
When asked to rate nonmusical sounds such as laughter and gasps, the Tsimane showed similar responses to the other groups. They also showed the same dislike for a musical quality known as acoustic roughness.
The findings suggest that it is likely culture, and not a biological factor, that determines the common preference for consonant musical chords, says Brian Moore, a professor of psychology at Cambridge University, who was not involved in the study.
“Overall, the results of this exciting and well-designed study clearly suggest that the preference for certain musical intervals of those familiar with Western music depends on exposure to that music and not on an innate preference for certain frequency ratios,” Moore says.

How the ‘police’ of the cell world deal with 'intruders’ and the 'injured’
The job of policing the microenvironment around our cells is carried out by macrophages. Macrophages are the 'guards’ that patrol most tissues of the body - poised to engulf infections or destroy and repair damaged tissue.
Over the last decade it has been established that macrophages are capable of detecting changes in the microenvironment of human tissues. They can spot pathogen invasion and tissue damage, and mediate inflammatory processes in response, to destroy microbial interlopers and remove and repair damaged tissue. But how do these sentinels of the cell world deal with infection and tissue injury?
Dr Anna Piccinini, an expert in inflammatory signalling pathways in the School of Pharmacy at The University of Nottingham, has discovered that the macrophage’s 'destroy and repair service’ is capable of discriminating between the two distinct threats even deploying a single sensor. As a result, they can orchestrate specific immune responses - passing on information in the form of inflammatory molecules and degrading tissue when they encounter an infection and making and modifying molecular components of the tissue when they detect tissue damage.
Dr Piccinini’s research is published today, Tuesday 30 August 2016, in the academic journal Science Signaling. Her findings could provide future targets for the treatment of diseases with extensive tissue damage such as arthritis or cancer where inflammation plays an increasingly recognized role.
Science Signaling
Macrophage Engulfing Bacteria, Artwork by David Mack



Why scientists are rooting for mushrooms
Mushrooms are the organisms that keep on giving. They grow and feed the soil by breaking down organic matter. For centuries, they’ve also been a staple in our diet.
Recently, people have started taking a closer look at mushrooms, and more specifically, mycelium — the hidden root of mushrooms — as an engineering material to produce goods like surfboards, packaging materials, furniture and even architecture.

As far as natural materials go, there’s never been anything as versatile and cost-effective as fungi, says Sonia Travaglini, a doctoral candidate in mechanical engineering at UC Berkeley, who is collaborating with artist and mycologist Philip Ross to unlock the seemingly infinite potential of fungi.
Mycelium can grow into any shape or size (the largest in the world blankets an entire forest in Oregon). They can be engineered to be as hard and strong as wood or brick, as soft and squishy as foam, or even smooth and flexible, like fabric.
Unlike other natural materials, mushrooms can rely on their recycling properties to break down organic matter so you can grow a lot of it very quickly and cheaply just by feeding it biodegradable waste. In as little as two weeks, you can cultivate a hunk of mushroom that’s brick-sized.
That mycelium actually takes in waste and carbon dioxide as it grows (one species of fungi even eats plastic trash) instead of expelling byproducts makes it far superior to other forms of production.
Plus, when you’re done with mushroom, you can compost it or break up the material to grow more mycelium from it.
“And, unlike forming synthetic materials, which have to be made while very hot or under pressure, all of which takes a lot of energy to create those conditions, mycology materials grow from mushrooms which grow in our normal habitat, so it’s much less energy-intensive,” said Travaglini.
In the lab, Travaglini and other researchers crush, compress, stretch, pull and bend mycelium to test the amount of force the material can tolerate.


They found that mycelium is incredibly strong and can withstand a lot of compression and tension.
Most materials are only strong from one direction. But mycology materials are tough from all directions and can absorb a lot force without breaking. So it can withstand as much weight as a brick, but won’t shatter when you drop it or when it experiences a hard impact, said Travaglini.
As one of the newer organisms receiving an application in biomimetics, a field of science that looks to imitate nature’s instinctive designs to find sustainable solutions and innovation, we might be getting merely a glimpse of what fungi is capable of.
“Mycology is still a whole new field of research, we’re still finding more questions and still really don’t know where it’s going to go, which makes it really exciting,” said Travaglini.
Image sources: Vice UK/Mazda & Pearson Prentice Hall

Collective memory in bacteria
Individual bacterial cells have short memories. But groups of bacteria can develop a collective memory that can increase their tolerance to stress. This has been demonstrated experimentally for the first time in a study by Eawag and ETH Zurich scientists published in PNAS.
Roland Mathis, Martin Ackermann. Response of single bacterial cells to stress gives rise to complex history dependence at the population level. PNAS, March 7, 2016 DOI: 10.1073/pnas.1511509113
Experimental set-up with the bacterium Caulobacter crescentus in microfluidic chips: each chip comprises eight channels, with a bacterial population growing in each channel. The bacteria are attached to the glass surface by an adhesive stalk. When the bacterial cells divide, one of the two daughter cells remains in the channel, while the other is washed out. Using time-lapse microscopy, bacterial cell-division cycles and survival probabilities can thus be reconstructed. Credit: Stephanie Stutz

I gotta split! Image of the Week - June 22, 2015
CIL:41466 - http://www.cellimagelibrary.org/images/41466
Description: Confocal image of a mitotic spindle in a dividing cell. The spindle is shown in yellow and the surrounding actin cytoskeleton is in blue. Sixth Prize, 2007 Olympus BioScapes Digital Imaging Competition.
Authors: Patricia Wadsworth and the 2007 Olympus Bioscapes Digital Imaging Competition®.
Licensing: Attribution Non-Commercial No Derivatives: This image is licensed under a Creative Commons Attribution, Non-Commercial, No Derivatives License

We might think we know the human body pretty well by now, but scientists are still discovering incredible individuals who are defying all odds by living out their lives with crucial parts missing, added, or tweaked in the most extraordinary ways.
From those with almost superhuman abilities, to others living without the organs we hold most dear, here are five of the most remarkable humans known to medicine.
Read more…


Meet the all-female team of coders that brought us Apollo 11.
In 1969, the world watched as Neil Armstrong marked his historic achievement with the words, “That’s one small step for man, one giant leap for mankind.” His now-famous transmission was heard around the globe thanks to NASA’s Deep Space Network, which made communication from outer space possible.
That network was built by a woman named Susan Finley. She was part of an all-female team of coders whose work was integral to the success of the Apollo 11 mission. Science writer Nathalia Holt brings us their stories in her book, Rise of the Rocket Girls: The Women Who Propelled Us from Missiles to the Moon to Mars.
Listen to their story here.
[Images via NASA]

A French man who lives a relatively normal, healthy life - despite missing 90 percent of his brain - is causing scientists to rethink what it is from a biological perspective that makes us conscious.
Despite decades of research, our understanding of consciousness - being aware of one’s existence - is still pretty thin. We know that it’s somehow based in the brain, but then how can someone lose the majority of their neurons and still be aware of themselves and their surroundings?
First described in The Lancet in 2007, the case of the man with the missing brain has been puzzling scientists for almost 10 years.
Read more…

A new study has found evidence that brain tumours use fat as their preferred source of energy, bringing into question the decades-long assumption that sugar is their main fuel source.
If confirmed, this could fundamentally change the we treat cancer in the future, because until very recently, scientists have been focussing their efforts on ways to starve cancer cells of their sugar supply.
“For 60 years, we have believed all tumours rely on sugars for their energy source, and the brain relies on sugars for its energy source, so you certainly would think brain tumours would,” lead researcher Elizabeth Stoll, a neuroscientist from Newcastle University in the UK, told Ian Johnston at The Independent.
Read more…










Giant Artwork Reflects The Gorgeous Complexity of The Human Brain
The new work at The Franklin Institute may be the most complex and detailed artistic depiction of the brain ever.
Your brain has approximately 86 billion neurons joined together through some 100 trillion connections, giving rise to a complex biological machine capable of pulling off amazing feats. Yet it’s difficult to truly grasp the sophistication of this interconnected web of cells.
Now, a new work of art based on actual scientific data provides a glimpse into this complexity.
The 8-by-12-foot gold panel, depicting a sagittal slice of the human brain, blends hand drawing and multiple human brain datasets from several universities. The work was created by Greg Dunn, a neuroscientist-turned-artist, and Brian Edwards, a physicist at the University of Pennsylvania, and goes on display at The Franklin Institute in Philadelphia.
“The human brain is insanely complicated,” Dunn said. “Rather than being told that your brain has 80 billion neurons, you can see with your own eyes what the activity of 500,000 of them looks like, and that has a much greater capacity to make an emotional impact than does a factoid in a book someplace.”
To reflect the neural activity within the brain, Dunn and Edwards have developed a technique called micro-etching: They paint the neurons by making microscopic ridges on a reflective sheet in such a way that they catch and reflect light from certain angles. When the light source moves in relation to the gold panel, the image appears to be animated, as if waves of activity are sweeping through it.
First, the visual cortex at the back of the brain lights up, then light propagates to the rest of the brain, gleaming and dimming in various regions — just as neurons would signal inside a real brain when you look at a piece of art.
That’s the idea behind the name of Dunn and Edwards’ piece: “Self Reflected.” It’s basically an animated painting of your brain perceiving itself in an animated painting.
To make the artwork resemble a real brain as closely as possible, the artists used actual MRI scans and human brain maps, but the datasets were not detailed enough. “There were a lot of holes to fill in,” Dunn said. Several students working with the duo explored scientific literature to figure out what types of neurons are in a given brain region, what they look like and what they are connected to. Then the artists drew each neuron.
Dunn and Edwards then used data from DTI scans — a special type of imaging that maps bundles of white matter connecting different regions of the brain. This completed the picture, and the results were scanned into a computer. Using photolithography, the artists etched the image onto a panel covered with gold leaf.
“A lot of times in science and engineering, we take a complex object and distill it down to its bare essential components, and study that component really well” Edwards said. But when it comes to the brain, understanding one neuron is very different from understanding how billions of neurons work together and give rise to consciousness.
“Of course, we can’t explain consciousness through an art piece, but we can give a sense of the fact that it is more complicated than just a few neurons,” he added.
The artists hope their work will inspire people, even professional neuroscientists, “to take a moment and remember that our brains are absolutely insanely beautiful and they are buzzing with activity every instant of our lives,” Dunn said. “Everybody takes it for granted, but we have, at the very core of our being, the most complex machine in the entire universe.”
Image 1: A computer image of “Self Reflected,” an etching of a human brain created by artists Greg Dunn and Brian Edwards.
Image 2: A close-up of the cerebellum in the finished work.
Image 3: A close-up of the motor cortex in the finished work.
Image 4: This is what “Self Reflected” looks like when it’s illuminated with all white light.
Image 5: Pons and brainstem close up.
Image 6: Putkinje neurons - color encodes reflective position in microetching.
Image 7: Primary visual cortex in the calcarine fissure.
Image 8: Basal ganglia and connected circuitry.
Image 9: Parietal cortex.
Image 10: Cerebellum.
Credit for all Images: Greg Dunn - “Self Reflected”
Source: The Huffington Post (by Bahar Gholipour)

A New Way to Cross the Blood–Brain Barrier - A Mental Unblock
The brain presents a unique challenge for medical treatment: it is locked away behind an impenetrable layer of tightly packed cells. Although the blood-brain barrier prevents harmful chemicals and bacteria from reaching our control center, it also blocks roughly 95 percent of medicine delivered orally or intravenously. As a result, doctors who treat patients with neurodegenerative diseases, such as Parkinson’s, often have to inject drugs directly into the brain, an invasive approach that requires drilling into the skull.
Some scientists have had minor successes getting intravenous drugs past the barrier with the help of ultrasound or in the form of nanoparticles, but those methods can target only small areas. Now neuroscientist Viviana Gradinaru and her colleagues at the California Institute of Technology show that a harmless virus can pass through the barricade and deliver treatment throughout the brain.
Gradinaru’s team turned to viruses because the infective agents are small and adept at entering cells and hijacking the DNA within. They also have protein shells that can hold beneficial deliveries, such as drugs or genetic therapies. To find a suitable virus to enter the brain, the researchers engineered a strain of an adeno-associated virus into millions of variants with slightly different shell structures. They then injected these variants into a mouse and, after a week, recovered the strains that made it into the brain. A virus named AAV-PHP.B most reliably crossed the barrier.
Next the team tested to see if AAV-PHP.B could work as a potential vector for gene therapy, a technique that treats diseases by introducing new genes into cells or by replacing or inactivating genes already there. The scientists injected the virus into the bloodstream of a mouse. In this case, the virus was carrying genes that encoded green fluorescent proteins. So if the virus made it to the brain and the new DNA was incorporated in neurons, the success rate could be tracked via a green glow on dissection. Indeed, the researchers observed that the virus infiltrated most brain cells and that the glowing effects lasted as long as one year. The results were recently published in Nature Biotechnology.
In the future, this approach could be used to treat a range of neurological diseases. “The ability to deliver genes to the brain without invasive methods will be extremely useful as a research tool. It has tremendous potential in the clinic as well,” says Anthony Zador, a neuroscientist who studies brain wiring at Cold Spring Harbor Laboratory. Gradinaru also thinks the method is a good candidate for targeting areas other than the brain, such as the peripheral nervous system. The sheer number of peripheral nerves has made pain treatment for neuropathy difficult, and a virus could infiltrate them all.
Image Credit: Thomas Fuchs
Source: Scientific American (By Monique Brouillette)

5 sleep disorders you didn’t know existed
Ever shouted at your partner while you slept, or woken up unable to move? From apnoea to exploding heads, here are some strange things that go bump in the night.
Sleep apnoea
A surprisingly common condition in which you stop breathing for 10 seconds or more as you sleep. The lack of oxygen causes your brain to wake you up, or pull you into much lighter sleep. Either way, it can have a profound effect on the quality of your sleep – and that of any bedfellow, as it’s often accompanied by loud snoring.
Sleep paralysis
A terrifying experience, where the body, which naturally becomes paralysed duringREM sleep, is still paralysed when you wake. You are fully conscious but cannot move or speak, sometimes for several minutes. Some people also feel as if they are choking or their chest is being crushed and they may have visual hallucinations. The condition can be exacerbated by sleep deprivation, some drugs, and disorders such as sleep apnoea.
Hypnagogic jerks
Those jumps or twitches you experience as you nod off, often accompanied by the sensation of falling. The cause remains a mystery. One idea is that you start dreaming before your body becomes paralysed. Another is that the twitches are a by-product of your nervous system relaxing as you drift off.
REM sleep disorder
If you’ve ever punched or shouted at your partner in the night, only to remember nothing next morning, you may have been in the grip of this condition. Here, the body isn’t fully paralysed during REM sleep, so people act out their dreams. Thistends to happen only with bad dreams.
Exploding head syndrome
This entails the sensation of a loud noise, like an exploding bomb or a gunshot, as you drift off or wake up. It affects about 1 in 10 of us and it tends to start around age 50. Nobody knows what causes it– perhaps physical changes in the middle ear, or a minor seizure in the brain’s temporal lobe. Despite its name, the condition is harmless.
Image Credit: Toby Leigh
Source: New Scientist (By Catherine de Lange)

After the four new additions, here’s a look at the origins of all the element names in the periodic table! High-res image/PDF: http://wp.me/p4aPLT-1Ru
Also featured in The Conversation UK alongside an article from Professor Mark Lorch here: https://goo.gl/g60pGU

Today is World Blood Donor Day – here’s a look at some blood chemistry! More info/high-res image: http://wp.me/s4aPLT-blood


The Red Hot Debate about Transmissible Alzheimer’s
In the 25 years that John Collinge has studied neurology, he has seen hundreds of human brains. But the ones he was looking at under the microscope in January 2015 were like nothing he had seen before.
He and his team of pathologists were examining the autopsied brains of four people who had once received injections of growth hormone derived from human cadavers. It turned out that some of the preparations were contaminated with a misfolded protein—a prion—that causes a rare and deadly condition called Creutzfeldt–Jakob disease (CJD), and all four had died in their 40s or 50s as a result. But for Collinge, the reason that these brains looked extraordinary was not the damage wrought by prion disease; it was that they were scarred in another way. “It was very clear that something was there beyond what you’d expect,” he says. The brains were spotted with the whitish plaques typical of people with Alzheimer’s disease. They looked, in other words, like young people with an old person’s disease.
For Collinge, this led to a worrying conclusion: that the plaques might have been transmitted, alongside the prions, in the injections of growth hormone—the first evidence that Alzheimer’s could be transmitted from one person to another. If true, that could have far-reaching implications: the possibility that ‘seeds’ of the amyloid-β protein involved in Alzheimer’s could be transferred during other procedures in which fluid or tissues from one person are introduced into another, such as blood transfusions, organ transplants and other common medical procedures.
Collinge felt a duty to inform the public quickly. And that’s what he did, publishing the study inNature in September, to headlines around the world. “Can you CATCH Alzheimer’s?” asked Britain’s Daily Mail, about the “potentially explosive new study”. Collinge has been careful to temper the alarm. “Our study does not mean that Alzheimer’s is actually contagious,” he stresses. Carers won’t catch it on the job, nor family members, however close. “But it raises concern that some medical procedures could be inadvertently transferring amyloid-β seeds.”
Since then, the headlines have died away, but the academic work and discussion have taken off. Could seeds of amyloid-β proteins really be transmitted and, if so, are they harmless or do they cause disease? And could seeds of other related diseases involving misfolded proteins be transmitted in a similar way? In the past decade or so, evidence has been mounting for a controversial theory that rogue proteins, known collectively as amyloids and associated with diverse neurodegenerative diseases—from Alzheimer’s to Parkinson’s and Huntington's—might share some properties of prions, including their transmissibility. Collinge’s data bolstered that theory.
Urgent though these questions are, it could take years to find answers. The paper by Collinge and his colleagues has sparked a worldwide hunt for similar amyloid pathology in autopsied brains, and a small study published in January 2016 revealed a handful of related cases. Researchers are also trying to work out what the putative amyloid seeds look like, and whether different 'strains’ of amyloids exist that are particularly damaging.
Some researchers say that it is much too early to be alarmed. They point out that the number of patients in Collinge’s study was tiny, that none had displayed symptoms of Alzheimer’s disease before their death and that another toxic protein called tau also seems to be required to cause the condition. “We have to remember that there is no conclusive evidence that seeds of amyloids can transmit actual disease or that amyloids spread in the brain in a prion-like way,” says Pierluigi Nicotera, scientific director of the German Centre for Neurodegenerative Diseases in Bonn. “There may be other biological explanations.”
Right now, there are few solid answers, but plenty of concerns. The sceptics worry that they might one day find themselves working under tight biosecurity regulations to handle proteins that they view as relatively innocuous. Others feel that the dangers may have been underestimated, and that scientists have a duty to investigate this as quickly as they can. “In my opinion, all amyloids should be considered dangerous until proven safe,” says prion and amyloid researcher Adriano Aguzzi at the University Hospital Zurich in Switzerland.
DANGEROUS FOLDS
A few decades ago, it was almost inconceivable that a protein, which has no genetic material or any other obvious way to self-replicate, could cause infectious disease. But that changed in 1982, when Stanley Prusiner, now at the University of California, San Francisco, introduced evidence for disease-causing prions, coining the term from the words 'proteinacious’ and 'infectious’. Prusiner showed that prion proteins (PrP) exist in a normal cellular form, and in a misfolded infectious form. The misfolded form causes the normal protein to also misfold, creating a cascade that overwhelms and kills cells. It cause animal brains to turn into a spongy mess in scrapie, a disease of sheep, and in bovine spongiform encephalopathy (BSE or 'mad cow disease’), as well as in human prion diseases such as CJD.
Prusiner and others also investigated how prions could spread. They showed that injecting brain extracts containing infectious prions into healthy animals seeds disease. These prions can be so aggressive that in some cases, simply eating infected brains is sufficient to transmit disease. For example, many cases of variant CJD (vCJD) are now thought to have arisen in the United Kingdom in the 1990s after people ate meat from cattle that were infected with BSE.
Since then, scientists have come to appreciate that many proteins associated with neurodegenerative diseases—including amyloid-β and tau in Alzheimer’s disease and α-synuclein in Parkinson’s disease—misfold catastrophically. Structural biologists call the entire family of misfolded proteins (including PrP) amyloids. Amyloid-β clumps into whitish plaques, tau forms ribbons called tangles and α-synuclein creates fibrous deposits called inclusions.
A decade ago, these similarities prompted neuroscientist Mathias Jucker at the University of Tübingen in Germany to test whether injecting brain extracts containing misfolded amyloid-β into mice could seed an abnormal build-up of amyloid in the animals’ brains. He found that it could, and that it also worked if he injected amyloids into the muscles. “We saw no reason not to believe that if amyloid seeds entered the human brain, they would also cause amyloid pathology in the same way,” says Jucker.
This didn’t cause alarm at the time, because it wasn’t clear how an amyloid seed from the brain of someone with Alzheimer’s could be transferred into another person’s body and find its way to their brain. To investigate that, what was needed was a group of people who had been injected with material from another person, and the opportunity to examine their brains in great detail, preferably when they were still relatively young and before they might have spontaneously developed early signs of Alzheimer’s.
The CJD brains provided just that opportunity. Between 1958 and 1985, around 30,000 people worldwide received injections of growth hormone derived from the adrenal glands of cadavers to treat growth problems. Some of the preparations were contaminated with the prion that causes CJD. Like all prion diseases, CJD has a very long incubation period, but once it gets going it rages through the brain, destroying all tissue in its wake and typically killing people from their late 40s onwards. According to 2012 statistics, 226 people around the world have died from CJD as a result of prion-contaminated growth-hormone preparations.
Collinge had not set out to find a link with Alzheimer's—it emerged as part of routine work at the National Prion Clinic in London, which he heads, and where around 70% of all people in the United Kingdom who die from prion-related causes are now autopsied. The clinic routinely looks for signs of all amyloid proteins in these brains to distinguish prion disease from other conditions. It was thanks to this routine work that the cluster of unusual cases emerged of people who had clearly died of CJD, but who also had obvious signs of amyloid pathology in their grey matter and cerebral blood vessels.
As soon as he saw these brains, Collinge knew that he could get into stormy waters. Keen to strike a balance between warning of a possible public-health risk and causing unwarranted panic, he sketched a carefully worded press release that would go out from the National Prion Centre and set up hotlines for people who had been treated with growth hormone in the past. But no panic occurred: apart from one or two overwrought headlines, the news stories were fairly measured, he says. Only around ten people called the hotlines.
For scientists, however, the paper was a red flag. “As soon as the paper came out we realized the health implications and started collecting slides and paraffin blocks from patients,” says Jiri Safar, director of the National Prion Disease Pathology Surveillance Center at Case Western Reserve University in Cleveland, Ohio. Like other pathologists in countries where people had died of CJD associated with medical procedures, he rushed to check the centre’s archives of autopsied brains to see if any of them contained the ominous amyloid deposits.
The answers are not yet in. Safar says that it has not proved easy to trace brain samples in the United States, but that he is working to do so with the National Institutes of Health and the Centers for Disease Control and Prevention (CDC) in Atlanta, Georgia. Charles Duyckaerts at the Pitié-Salpêtrière Hospital in Paris, France, has now examined brain tissues from around 24 patients and is likely to report the results later this year.
A further 228 cases of CJD were caused by transplantation of prion-contaminated dura mater—the membrane surrounding the brain and spinal cord—prepared from cadavers around the world. Dura-mater preparations were regularly used in brain surgery as repair patches until the late 1990s. For the study published in January, Herbert Budka at the National Prion Diseases Reference Center at University Hospital Zurich and his colleagues examined the brains of seven such patients from Switzerland and Austria, and found that five had amyloid deposits in grey matter and blood vessels. In Japan, dementia researcher Masahito Yamada at Kanazawa University is making his way through a large number of such autopsy specimens and says that the 16 brains he has examined so far show signs of unusually high levels of amyloid deposition in cerebral blood vessels.
Yet such case studies can only ever provide circumstantial evidence that seeds of amyloid-β were transferred during the treatments. And they cannot entirely rule out the possibility that the treatments themselves—or the patients’ original medical conditions—caused the amyloid pathology. More-conclusive evidence would come from checking whether the original growth hormone and dura-mater preparations contained infectious amyloid seeds, by injecting them into animals and seeing whether this triggers disease. Most of these preparations, however, have long since disappeared. Collinge has access to some original samples of growth hormone stored by the UK Department of Health, and he is planning to analyse them for the presence of amyloid seeds and then inject them into mice. That work will take a couple of years to complete, he says.
SEEDS OF DOUBT
There is another hitch: no one knows for sure what size and shape the amyloid seeds might be. Jucker is hunting for them in an unusual source of human brain tissue that has nothing to do with CJD. A team in Bonn has collected frozen samples from more than 700 people with epilepsy who were operated on over the past 25 years to remove tissue that was driving their seizures. “It is the best source of fresh human brain tissue available at the moment,” says Jucker, who plans to scrutinize it carefully under the microscope for anything that might resemble tiny clumps or seeds of amyloid-β. The team also has records of the patients’ cognitive skills, such as language and memory skills, before and at regular intervals after the operations. This should allow Jucker’s team to correlate the presence of any amyloid-β seeds it finds with changes in the cognitive function of individual patients over time.
Scientists have shown that tau and α-synuclein can also seed pathological features in mice. In two studies, from 2012, scientists injected fibrils of α-synuclein into the brains of mice already engineered to develop some of the characteristics of Parkinson’s disease. This triggered the early onset of some of the signs and symptoms of Parkinson’s, and eventually killed the animals. A third study showed that similar injections into normal mice caused some of the neurodegeneration typical of Parkinson’s disease and the mice became less agile. In humans, α-synuclein would not necessarily turn out to be equally aggressive—mouse models of neurodegenerative diseases do not mimic human disease very closely—but scientists are taking the possibility seriously.
If the transmissibility hypothesis proves true, the implications could be severe. Amyloids stick like glue to metal surgical instruments, and normal sterilization does not remove them, so amyloid seeds might possibly be transferred during surgery. The seeds might sit in the body for years or decades before spreading into plaques, and perhaps enabling the other pathological changes needed to induce Alzheimer’s disease. Having amyloid plaques in cerebral blood vessels could be dangerous in another way, because they increase the risk that the vessel walls might break, leading to small strokes.
But if common medical procedures really increased the risk of neurodegenerative disorders, then wouldn’t that already have been detected? Not necessarily, says epidemiologist Roy Anderson at Imperial College London. “The proper epidemiological studies have not been done yet,” he says. They require very large and carefully curated databases of people with Alzheimer’s disease, which include information about the development of symptoms and autopsy data. He and his team are now studying the handful of reliable databases that exist to tease out a signal that might associate medical procedures with Alzheimer’s progression. The number of patients currently available may turn out to be too small to draw conclusions, he says, but a more definitive answer could emerge as the databases grow.
Faced with so much uncertainty, some researchers and public-health agencies have adopted a wait-and-see approach. “We are right at the beginning of this story,” says Nicotera, “and if there is one message to come out right now it is that we need more work to see if this is a relevant mechanism.” The CDC and the European Centre for Disease Prevention and Control in Solna, Sweden, say that they are keeping a cautious eye on the issue.
If further research does confirm that common neurodegenerative diseases are transmissible, what then? One immediate priority would be rigorous sterilization procedures for medical and surgical instruments that would destroy amyloids, in the way that extremely high temperatures and harsh chemicals destroy prions. Aguzzi says that funding agencies should put out calls now to researchers to develop cheap and simple sterilization methods. “It’s not very sexy science, but it is urgently needed,” he says. He also worries about the safety of researchers working with amyloids—particularly α-synuclein. “I have nightmares that someone in my lab may catch Parkinson’s,” he says. “While the story is in flux, our first duty is to protect lab workers.”
STRAIN SEEKERS
The similarities between prions and other amyloids is throwing open other avenues of research. Prions can exist as distinct strains—proteins that have the same sequence of amino acids but misfold in different ways and have distinct biological behaviours, much as different strains of a pathogenic virus can be aggressive or weak. The outbreak of vCJD in the United Kingdom in the 1990s was traced to BSE-contaminated meat because the prion strain was the same in both.
Over the past few years, research in animals has shown that different strains of amyloid-β and α-synuclein exist. And a landmark paper in 2013 reported that strains of amyloid-β with different 3D structures were associated with different disease progression in two people with Alzheimer’s. Structural biologist Robert Tycko, who led the work at the National Institute of Diabetes and Digestive and Kidney Diseases in Bethesda, Maryland, is now looking at many more brain samples from such patients.
Knowing the structures of pathological forms of amyloid seeds should help to design small molecules that bind to them and stop them doing damage, says biophysicist Ronald Melki at the Paris-Saclay Institute of Neuroscience, who works on α-synuclein strains. His lab is designing small peptides that target the seeds and mimic regions of 'chaperone’ molecules, which usually bind to proteins and help them to fold correctly. Melki’s small peptides mimic these binding regions, sticking to the amyloid proteins to stop them from aggregating further.
In the research community, much of the agitation in response to Collinge’s paper boils down to semantics. Some scientists do not like to use the word 'prion’ in connection with the amyloids associated with common neurodegenerative diseases, or to describe any of their properties as 'prion-like'—because of its connotation of infectious, deadly disease. “The public has this perception of the word 'prion’,” says Alzheimer’s researcher Brad Hyman at Harvard Medical School in Boston, Massachusetts, and this matters, even if their ideas are wrong. “One of my patients told me that she wasn’t getting any hugs any more from her husband who had read about the case in the media—that made me sad,” he says.
Others, however, feel that it is helpful to consider prions and other amyloids as being part of a single spectrum of conditions involving proteins that misfold and misbehave. It means that researchers studying prion diseases and neurodegenerative diseases, who until recently had considered their disciplines to be separate, now find themselves tackling shared questions.
Both fields are wary of raising premature alarm, even though they wonder what the future will bring. Jucker, only half-jokingly, says he could imagine a future in which people would go into hospital every ten years or so and get the amyloid seeds cleared out of their brains with antibodies. “You’d be good then to go for another decade.”
Image 1 Credit: ©iStock.com
Image 2 Credit: Juan Gaertner/Shutterstock
Source: Scientific American (By Alison Abbott, Nature magazine)




Today is the International Day of Women and Girls in Science, so let’s write women back into science history. Check out the gallery here.

Autonomic nervous system
Structure and Function of the Sympathetic and Parasympathetic nervous system
The main function of the autonomic nervous system (ANS) is to assist the body in maintaining a relatively constant internal environment. For example, a sudden increase in systemic blood pressure activates the baroreceptors (those are receptors that detect physical pressure) which in turn modify the activity of the ANS so that the blood pressure is restored to its previous level [1].
The ANS is often regarded as a part of the motor system and is responsible for involuntary action and its effector organs are smooth muscle, cardiac muscle and glands. Another system, the somatic (meaning around the body) nervous system, is responsible for voluntary action in which skeletal muscle is the effector.
The ANS can further be divided into 3 parts: sympathetic, parasympathetic and enteric nervous systems [1][2], with the enteric nervous system sometimes being considered a separate entity [2]. Both parasympathetic and sympathetic nervous systems coexist and work in opposition with each other, ultimately maintaining the correct balance; the activity of one being more active depending on the situation. In a normal resting human, the parasympathetic nervous system dominates, while in a tense and stressful situation, the sympathetic nervous system switches to become dominant.

Figure 1. Structure and function of the central nervous system
This article will be focused on sympathetic and parasympathetic activity from the perspective of:
Anatomy
Biochemical
The sympathetic division provides your “fight or flight” whereas the parasympathetic division helps you to “rest and digest”
Anatomy
Higher centers that control autonomic function include the pons, medulla oblongata and hypothalamus [3].
The pons contains the micturition (urination) and respiratory center.
The medulla oblongata contains the respiratory, cardiac, vomiting, vasomotor and vasodilator centres [4].
The hypothalamus contains the highest concentration of autonomic centres [4]. It contains several centres that control autonomic activities, including heat loss, heat production and conservation, feeding and satiety, as well as fluid intake [4].

Figure 2. Locations of the autonomic control centres of the brain
All 3 structures receive input from certain sources by stimulation of nerve fibres resulting from chemical changes in blood composition like blood pH, blood glucose level, blood osmolarity and volume [4]. Notably, the hypothalamus receives input from cerebral cortex and the limbic system, a system that helps control emotional behaviour [3].
Autonomic promoter neurons are neurons that are found in the brain stem, hypothalamus or even cerebral hemispheres that project to preganglionic neurons (discussed below), where they form synapses with these neurons (5). Hence, input from the higher centres can be relayed to the motor neurons (preganglionic and then postganglionic neurons) which subsequently innervate different body tissues. Changes in the input from these centres could result in responses in those tissues.
The primary functional unit of the sympathetic and parasympathetic nervous system consists of a 2 neuron motor pathway (Figure 3), containing a preganglionic and postganglionic neurons, arranged in series.(2) The two synapse in peripheral ganglion. This clearly distinguishes autonomic motor nervous system and somatic nervous system. The somatic nervous system project from the CNS directly to innervated tissue without any intervening ganglia.(6)

Figure 3. Diagram showing the primary functional unit of the ANS
Sympathetic nervous system
Sympathetic preganglionic neurons mainly are concentrated in the lateral horn in the thoracic (T1-12) and upper lumbar (L1 &2) segments of the spinal cord (Figure 4).
The preganglionic axons leave the spinal cord in 3 ways:
Through the paravertebral ganglion
The preganglionic axon may synapse with postganglionic neurons in this ganglion or some axon may travel rostrally or caudally within the sympathetic trunk before forming synapse with a postganglionic neurons in a different paravertebral ganglion.
Through the prevertebral ganglion
Some preganglionic axons pass the paravertebral ganglion (no synapse occur) and form synapse with postganglionic neurons in prevertebral ganglion, also known as collateral ganglion.
Directly to the organs without any synapse
Some preganglionic axons pass through the sympathetic trunk (no synapse) and end directly on cells of the adrenal medulla, which are equivalent to postganglionic cell.
Parasympathetic nervous system
The parasympathetic preganglionic neurons are located in several cranial nerve nuclei in the brain stem and some are found in the S3 and S4 segments of the sacral spinal cord (Figure 4). The parasympathetic postganglionic neurons are located in cranial ganglia, including the ciliary ganglion, the pterygopalatine, submandibular ganglia, and the otic ganglion. Other ganglia are present near or in the walls of visceral organs. Similarly, the preganglionic neurons form synapse with the postganglionic neurons in the ganglia.

Figure 4. Anatomy of the ANS and how its nuerons innervate tissues
After knowing how nerves connect from the CNS to PNS and to different organs, we will now consider some of the neurotransmitters that are being released at different nerve terminals. It is the binding of these neurotransmitters to the receptors on the effectors that leads to biochemical and physiological changes. Some of the neurotransmitters in use are:
For the synapse between pre and postganglionic neurons mentioned above, the neurotransmitter that is released by the preganglionic axon terminal, is acetylcholine. The corresponding receptors are found on the postsynaptic membrane of postganglionic nerves and are nicotinic receptors.
Parasympathetic postganglionic nerve terminals also release acetylcholine.
Sympathetic postganglionic nerve terminals release mostly noradrenaline
The adrenal medulla receives direct stimulation from sympathetic preganglionic innervation, releases mainly adrenaline (80%) and some noradrenaline into the blood stream. In this case, both adrenaline and noradrenaline act as hormones as they are transported via blood circulating system to target organs instead of neuronal pathway.
Strangely, for the sympathetic postganglionic nerves that innervate the sweat glands, the nerves release acetylcholine (normally only by parasympathetic postganglionic nerve) instead.
1. H.P.Rang, J.M.Ritter, R.J.Flower GH. RANG & DALE’S Pharmacology. In: 8th ed. ELSEVIER CHURCHILL LIVINGSTONE; 2016. p. 145.
2. Bruce M. Koeppen BAS. BERNE & LEVY PHYSIOLOGY. In: 6th ed. MOSBY ELSEVIER; 2010. p. 218.
3. Cholinergic transmission [Internet]. 2015. Available from: http://www.dartmouth.edu/~rpsmith/Cholinergic_Transmission.html
4. Bruce M. Koeppen BAS. BERNE & LEVY PHYSIOLOGY. In: 6th ed. MOSBY ELSEVIER; 2016. p. 44.

It’s a tremendous Trilobite Tuesday!
When most of us think about trilobites, we imagine rather small creatures that inhabited the ancient seas. Indeed, most members of the more than 25,000 scientifically recognized trilobite species were less that three inches in length. Occasionally, however, paleontologists encounter a megafauna where, due to a variety of circumstances, the trilobite species were huge. One of these megafaunas can be found near the small Portuguese town of Arouca where the 450 million year-old Valongo formation produces prodigious numbers of exceptionally large Ordovician-age trilobites, such as this 41 cm Hungioides bohemicus. Other trilobite magafaunas appear sporadically around the globe, including Cambrian locations in Morocco and Devonian outcrops in Nevada.
Meet many more trilobites on the Museum website.

Heart Regeneration
If a person suffers a heart attack and survives, chances are their heart muscle will never be quite the same. Indeed, the associated scarring often results in permanent damage that can lead to heart failure and eventual death. Scientists are therefore searching for possible ways to promote regeneration of damaged hearts, and it’s possible that newborn mice may hold the answer. For a few weeks after birth, these animals can almost entirely regenerate their heart tissue after an injury. And new research suggests a key process that may be critical for this regenerative ability: regrowth of nerves. Blocking nerve growth specifically in experimental animals completely prevented the regrowth of damaged heart tissue. In control animals, the nerves regrew into their normal branching patterns—like those pictured. Thus if researchers are to have any hope of regenerating adult hearts after injury, their best bet might be to boost accompanying nerve growth.
Written by Ruth Williams
Image from work by Ian A. White and colleagues
Interdisciplinary Stem Cell Institute, University of Miami Miller School of Medicine, USA
Image copyright held by the American Heart Association
Published in Circulation Research, December 2015
You can also follow BPoD on Twitter and Facebook

This Week in Chemistry: Preventing marble statue weathering, further progress towards hydrogen fusion, and more! Links: http://goo.gl/WeJRV5

After the news of an accident in a French drug trial on Friday, you might be wondering what drug trials entail. Here’s a summary sheet on the drug discovery process to clear things up! http://wp.me/p4aPLT-1EZ

Scientists have developed a new drug that could be a safer alternative to morphine for medical use. The researchers found that engineered variants of endomorphin, a naturally occurring chemical in the body, are as strong as morphine when it comes to killing pain.
On top of that, the medication doesn’t produce any of the unwanted side effects that come with opium-based drugs – such as being extremely addictive. At this point, the findings only relate to tests in rats, but it’s a promising start to what could be a powerful and less problematic painkiller.
Opioid pain medications are commonly used to treat severe and chronic pain, but in addition to their habit-forming qualities, patients also build up a tolerance to them over time. Hand in hand with their addictiveness, this can makes higher doses – and overdoses in drug abuse situations – dangerous. Overdoses can cause motor impairment and potentially fatal respiratory depression, resulting in thousands of deaths in the US every year.
Steve Gentleman, a neuropathologist, demonstrates the process of brain dissection and preservation for research.

New insights into the molecular basis of memory
Scientists from the German Center for Neurodegenerative Diseases (DZNE) in Göttingen and Munich have shed new light on the molecular basis of memory. Their study confirms that the formation of memories is accompanied by an altered activity of specific genes. In addition, they found an unprecedented amount of evidence that supports the hypothesis that chemical labels on the backbone of the DNA (so-called DNA methylation) may be the molecular basis of long-term memory. These findings are reported in “Nature Neuroscience”.
The brain still harbours many unknowns. Basically, it is assumed that it stores experiences by altering the connections between brain cells. This ability to adapt – which is also called “plasticity” – provides the basis for memory and learning, which is the ability to draw conclusions from memories. On a molecular scale these changes are mediated by modifications of expression of specific genes that as required strengthen or weaken the connections between the brain cells.
In the current study, a research team led by Dr. Stefan Bonn and Prof. André Fischer from Göttingen, joined forces with colleagues from the DZNE’s Munich site, to examine how the activity of such genes is regulated. The scientists stimulated long-term memory in mice, by training the animals to recognise a specific test environment. Based on tissue samples, the researchers were able to discern to what extent this learning task triggered changes in the activity of the genes in the mice’s brain cells. Their focus was directed on so-called epigenetic modifications. These modifications involve the DNA and DNA associated proteins.
Epigenetic modifications
“The cell makes use of various mechanisms in order to turn genes on or off, without altering the DNA sequence itself. It’s called ‘epigenetics’,” explains Dr. Magali Hennion, a staff member of the research group of Stefan Bonn.
In principle, gene regulation can happen through methylation, whereby the backbone of the DNA is chemically labeled at specific sites. Changes in the proteins called histones that are packaging the DNA may also occur.
Hennion: “Research on epigenetic changes that are related to memory processes is still at an early stage. We look at such features, not only for the purpose of a better understanding of how memory works. We also look for potential targets for drugs that may counteract memory decline. Ultimately, our research is about therapies against Alzheimer’s and similar brain diseases.“
A code for memory contents?
In the current study the researchers found modifications, both of the histones as well as of the methylation of the DNA. However, histone modifications had little effect on the activity of genes involved in neuroplasticity. Furthermore, Bonn and his colleagues not only discovered epigenetic modifications in nerve cells, but also in non-neuronal cells of the brain.
“The relevance of non-neuronal cells for memory, is an interesting topic that we will continue to pursue“, says André Fischer, site speaker for the DZNE in Göttingen and professor at the University Medical Center Göttingen (UMG). “Furthermore, our observations suggest that neuroplasticity is to a large extent regulated by DNA methylation. Although this is not a new hypothesis, our study provides an unprecedented amount of supporting evidence for this. Thus, methylation may indeed be an important molecular constituent of long-term memory. In such a case, methylation could be a sort of code for memory content and a potential target for therapies against Alzheimer’s disease. This is an aspect that we specifically want to focus on, in further studies.”










TOP TEN MOST DEADLY INFECTIOUS DISEASES
This list is based off of the assumption that the infected individual does not receive medical treatment.
1. Prions (mad cow disease, Creutzfeld-Jakob disease, kuru, fatal familial insomnia): 100%
2. Rabies: ~100%
3. African trypanosomiasis (’African sleeping sickness’): ~100%
4. Primary amoebic encephalitis caused by Naegleri fowlerii (’the brain-eating amoeba’): ~100%
5. Yersinia pestis, specifically the pneumonic or septicemic subtype (’the black plague’): ~100%
6. Visceral leishmaniasis: ~100%
7. Smallpox, specifically the malignant (flat) or hemorragic subtype: 95%
8. Ebola virus, specifically the Zaire strain: 83-90%
9. HIV: 80-90%
10. Anthrax, specifically the pulmonary subtype: >85%

4 new elements added to the periodic table
The seventh row of the Periodic Table of Elements is now complete, rendering all textbooks out of date. The discovered elements don’t have permanent names yet, but their atomic numbers are 113, 115, 117 and 118.
Livermore Lab scientists and international collaborators have officially discovered three of the four new elements: 115, 117 and 118. The illustration above is of 117, tentatively named ununseptium or Uus.
The new elements’ existence was confirmed by further experiments that reproduced them — however briefly. Element 113, for instance, exists for less than a thousandth of a second.
Learn more about the new elements