Dissecting Iron Man Suit - An Engineering Analysis
Dissecting Iron Man Suit - An Engineering Analysis
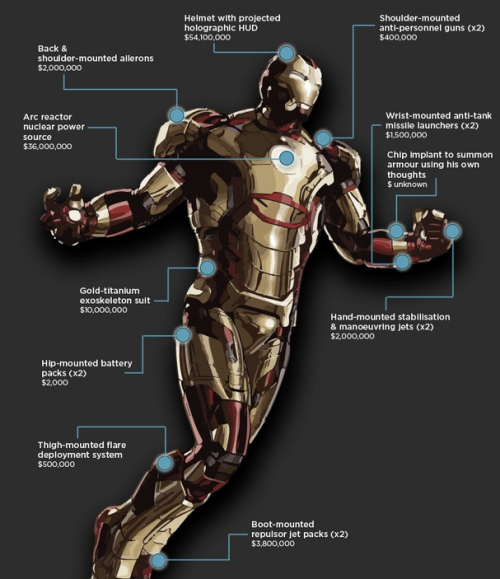
Structural, energy, and thermal analysis of Iron Man Suits specifically Mark I to Mark XLVI which have the following capabilities in common: external armor, supersonic flight, hovering, weaponry, and decoy flares.
1. STRUCTURAL ANALYSIS
Wear Resistant and Shock Absorbent Exoskeleton The physical protective value of exoskeleton is its ability to resist any penetrative loads as well as any shock loads. However, the whole thickness of exoskeleton panels should not be too hard because it will pass on the external impact load into the suit’s internal hardware, or even the human body inside it. All of this can be achieved by combining more than one materials; a hard material on the outside and the soft material on the inside
Hard Outer Layer for Penetrative Loads The materials needed for the exoskeleton’s outer layer should be hard and tactile. Titanium Alloy would be an ideal choice. Fiber glass has good tensile strength but not good shear strength, while titanium has both .Titanium Alloy is not only much stronger, but is also lighter than steel, which will provide more fluidity of movement compared to any heavy material counterparts.
Ductile Inner Layer for Shock Loads There should be a soft inner linings behind titanium panels to serve as shock absorbent. Sorbothane is a material that is extremely soft and has the ability to convert shock loads into heat transfer at a molecular level. It is a proprietary, visco-elastic polymer. Visco-elastic means that a material exhibits properties of both liquids (viscous solutions) and solids (elastic materials).
Sorbothane is a thermoset, polyether-based, polyurethane material. Sorbothane combines shock absorption, good memory, vibration isolation and vibration damping characteristics. In addition, Sorbothane is a very effective acoustic damper and absorber. Even if one drops an egg from the top of a building into a bed of sorbothane, this remarkable material is soft enough to cushion the impact and would not allow the egg to break.
This technique of having a hard material on the outside and the soft material on the inside is not new. It has been used for centuries in Japan for making samurai swords. The hardness of its outer layer give the swords its cutting edge and penetrative power, and its ductility allows it to absorb shock loads when it strikes or struck.
2. ENERGY ANALYSIS : Hovering Capability
Hovering using thrusters (aka repulsors) requires tremendous amount of energy, particularly when the suit is used for a long duration. Energy usage for hovering is dependent upon the hovering methods
Magnetic Levitation requires no energy at all, but is limited to the presence of magnetic field.
Ducted and Open Propellers (helicopter blades). Several human powered helicopters have been made overtime that have achieved flight. It has been experimentally recorded that a 78 kg person in a 58 kg copter requires only 1.1 kW to climb using helicopter blades, and only 60 Watts to maintain altitude.
Jet Thrust is the least energy-efficient method. Because thrust-to-weight ratio needs to be greater than 1 to achieve lift-off, a Jet-pack requires over 1KN of thrust force, depending on the weight of the jet and the person. If wings are attached to the jet-pack, horizontal flight can be achieved with thrust to weight ratio lower than 1, thus improving the duration of the flight and its range.There have been jet-packs made in the past, most iconic display of it was in 1994 Olympics opening ceremony. The fuel used in the jet-pack was mostly hydrogen peroxide. It provides thrust at low temperature compared to other fuels. However, it has low energy density of 810 Wh/kg, giving the jet-packs up to only 30 seconds of flight-time. Jet’s flight time is limited even by using energy-rich fossil fuel. Yves Rossy (aka Jet Man) has successfully used kerosene oil in his flight, but the thruster jets have to be pushed away from the body for safety. His suit allows only several minutes of flight. In addition, if a heavier suit (greater than 25 kg) is used, hydraulics are needed, which would require additional energy and slow down mobility. The Iron Monger suit was an example of hydraulic-driven mobility suit.
3. POWER SOURCE
Tony Stark manages the suit’s energy requirements, including thermal management and artificial intelligence system, through the fictional arc reactor. The reactor is able to provides almost limitless clean energy despite being a very small device. In real life, the only thing that has an energy density comparable to the arc reactor, and would meet all the energy requirements of the suit would be nuclear power. Uranium (fission) energy density is 80.620.000 MJ/kg. However, nuclear power is not suitable to be harnessed in a manned suit, since it generates a tremendous amount of heat.
A more practical solution would be a battery energy-storage. If lithium batteries are used on propeller blades, minutes-long flight time can be achieved. Furthermore, these batteries can readily power suit’s electrical devices / electronics requirements. Lithium ion battery has energy density of 150 Wh/kg (0.5 MJ/kg). Fossil fuel, on the other hand, have a much higher energy density than batteries, but would require a clunky generator to power the suit’s electrical requirements.
Lithium sulfur batteries have 5 times more energy density compared to lithium ion batteries. Lithium sulfur packs had already powered the longest unmanned flight for more than 30 hours. Unless we discover something like an arc-reactor, lithium sulfur batteries could be just the thing to power up the suit. The downside is, it requires hours of charging for just minutes of usage.
There is an alternative option, though not a ‘reactor’ proper. A compact and high-output generator (standard car alternators crank out 50-70 amps at 12 volts for years, and some can go as high as 150 amps) could be spun by a small and strong output electric motor (all alternators have to do is spin). That motor can be powered by a high density battery like used for electric bikes in the 1500w to 2500w range at 20 something volts. This would power a strong and small motor at 3500 to 4000 rpm for hours. That’s more than enough to create power for a number of systems, if they’re built to take advantage of the amperage. And with new constructions of carbon arrays coming out every day, one or more of those could bring a meaningful electric output increase in an otherwise standard generator, even above what we have in cars now.
4. THERMAL MANAGEMENT
The suit cannot be hermetically sealed. Human body heat evaporates water from the skin. Therefore, air ventilation is a must to remove them. It is also needed to maintain a good supply of oxygen. So, there must be a structure inside the exoskeleton that allows air flow. This would prevent any internal condensation to settle and will also remove buildup of body heat. The layer of sorbothene would act both as a thermal and an electrical insulator. This means that extreme external temperature would not be transferred to the inner layer. The suit would not get too hot or too cold from the outer environment. There should be small fans to draw and pull air from the ambient in controlled amount, and should be able to exchange hot air. With the technology available today, the thermal management of the suit is easily manageable. There are also solid state devices such as thermal pads and thermoelectric generators. Thermoelectric generators can surfaces hot or cold depending on the polarity of the electric current and thus can be an integral component of the suit for controlling the internal temperature.
Source (x)
Keep reading
More Posts from T-sci-eng and Others



Superstretchable, supercompressible supercapacitors
Flexible, wearable electronics require equally flexible, wearable power sources. In the journal Angewandte Chemie, Chinese scientists have introduced an extraordinarily stretchable and compressible polyelectrolyte which, in combination with carbon nanotube composite paper electrodes, forms a supercapacitor that can be stretched to 1000 percent in length and compressed to 50 percent in thickness with even gaining, not losing capacity.
Supercapacitors bridge the gap between batteries, which are merely energy-storing devices, and normal capacitors, which release and take up electric energy very quickly but cannot store so much energy. With their ability to charge and release large amounts of electric power in a very short time, supercapacitors are preferably used in regenerative braking, as power buffers in wind turbines, and, increasingly, in consumer electronics such as laptop computers and digital cameras. To make supercapacitors fit for future electrics demands like, for example, wearables and paper electronics, Chunyi Zhi from the City University of Hong Kong and his colleagues are searching for ways to endow them with mechanical flexibility. It can be achieved with a new electrolyte material: they developed a polyelectrolyte that can be stretched more than 10 times its length and compressed to half its thickness retaining full functionality, without breakage, cracking, or other damage to its material.
Read more.

This room starts charging your phone as soon as you walk in. Inspired by Tesla’s vision of global wireless power, scientists at Disney Research company explored how wireless charging works in large spaces. The copper pole at the room’s center sends currents through the walls and floor that charge phones and laptops without harming humans. Source Source 2

Devices can be charged regardless of their orientation in the room thanks to a new receiver design

The setup outside the room

The setup inside the room

How did the Greeks know ?
Greeks had a strong geometric approach towards problems and as a result their methods are very intuitive.
In this post, we will look at the Method of exhaustion formulated by Archimedes that stands out as a milestone in the history of mathematics
Method of Exhaustion - Archimedes

Source
In order to find the bounds of pi, Archimedes came up with a remarkably elegant ‘algorithm’, which is as follows:
Lower bound
Inscribe a n-sided polygon in a circle —> Measure its perimeter(p) —> Measure its diameter(d) —> pi_min = p/d —-> Repeat with n+1 sides.
Upper bound
Circumscribe a n-sided polygon in a circle —> Measure its perimeter(p) —> Measure its diameter(d) —> pi_max = p/d —-> Repeat with n+1 sides.
And by following this procedure one could obtain the upper and lower bounds of pi !
Heres an animation made on geogebra for a circle of diameter 1. Watch how the lower and upper bounds vary.

Archimedes did this for a 96 sided polygon and found the value of pi to be between 3.14103 and 3.1427. This is a good enough approximation for most of the calculations that we do even today!

Happy Holidays !




Alloys: Wood’s Metal
Also known as Lipowtiz’s alloy as well as the commercial names of Cerrobend, Bendalloy, Pewtalloy, and MCP 158 among others, Wood’s metal is a bismuth alloy consisting of 50% bismuth, 26.67% lead, 13.33% tin, and 10% cadmium by weight. Named for the man who invented it, a Barnabas Wood, Wood’s metal was discovered/created by him in 1860.
Wood’s metal is both a eutectic and a fusible alloy, with a low melting temperature of approximately 70 °C (158 °F). While none of its individual components have a melting temperature of less than 200 °C, a eutectic alloy can be considered as a pure (homogeneous) substance and always has a sharp melting point. If the elements in a eutectic compound or alloy are not as tightly bound as they would be in the pure elements, this leads to a lower melting point. (Eutectic substances can have higher melting points, if its components bind tightly to themselves.)
Useful as a low-temperature solder or casting metal, Wood’s metal is also used as valves in fire sprinkler systems. Thanks to its low melting temperature, Wood’s metal melts in the case of a fire and thanks to the bismuth it is made from, the alloy also shrinks when it melts (bismuth, like water ice, is one of the few substances to do so) which is the key to setting off the sprinkler system. Wood’s metal is also often used as a filler when bending thin walled metal tubes: the filler prevents the tube from collapsing, then can be easily removed by heating and melting the Wood’s metal. Other applications include treating antiques, as a heat transfer medium in hot baths, and in making custom shaped apertures and blocks for medical radiation treatment.
With the addition of both lead and cadmium, however, Wood’s metal is considered to be a toxic alloy. Contact with bare skin is thought to be harmful, especially once the alloy has melted, and vapors from cadmium containing alloys are also quite dangerous and can result in cadmium poisoning. A non-toxic alternative to Wood’s metal is Field’s metal, composed of bismuth, tin, and indium.
Sources: ( 1 - image 4 ) ( 2 - image 2 ) ( 3 ) ( 4 )
Image sources: ( 1 ) ( 3 )




Ceramics: Aluminum Nitride
First synthesized in the late 1800s, aluminum nitride’s potential wasn’t realized until a hundred years later in the late 1900s. AlN is a ceramic with high thermal conductivity but is an electrical insulator. It is classified as a covalent compound, the only stable compound in the binary Al-N system.
AlN is similar in properties to beryllium oxide (BeO), but is cheaper and has less of a potential to be toxic. In addition to the properties mentioned above, this ceramic also has high chemical resistance and exhibits piezoelectric properties.
Thanks to its thermal and electrical conductivity properties, AlN is useful in microelectronics. It is used in microelectronic packaging, surface acoustic wave sensors, in RF filters, as a crucible for the growth of gallium arsenide crystals, in piezoelectric MEMs applications, and many more. In addition, the wurtzite phase of aluminum nitride, w-AlN, is a wide band gap semiconductor material, with potential applications in deep ultraviolet optoelectronics.
Because AlN is a covalent compound, high pressures or sintering aids are required to assist densification during production. Typical additives include rare-earth or alkaline-earth oxides, such as yttrium compounds. The additives and sintering conditions used can alter the properties of commercially available grades of AlN.
Sources: ( 1 ) ( 2 - images 2 and 3 ) ( 3 - images 1 and 4 ) ( 4 )

Adieu 2016 - Best of FYP!
2016 has been a great year for FYP!
And we would like to conclude it with some of the best posts that we have been able to produce
1. Black hole are not so black - series

Part - I , II, III
2.‘Katana’ - A sword that can slice a bullet

3. A denied stardom status - Jupiter

4. The Pythagoras Cup

5. On Pirates and Astronomers

6. Behold- The Space Shuttle Tile

7. Principle of Least Effort

8. Leidenfrost Effect

9. Major Types of Engines

10. A holy matrimony of Pascals and Sierpinski’s Triangle

11. Curves of constant width

12. Smooth Ride, Bumpy Road

Thank you so much following us ! Have a great weekend :D
- Fuck Yeah Physics!

Møbius donut.
How platinum powers the world

Platinum bars. Image: Sprott Money@Flickr
Platinum is one of the most valuable metals in the world. Precious and pretty, it’s probably best known for jewelry – and that is almost certainly its oldest use. But its value has become far greater than its decorative ability; today, platinum powers the world. From agriculture to the oil markets, energy to healthcare, we use platinum far more than we realise.
1. Keep the car running

Platinum is needed to make fuel for transport. Image: Pixabay
Platinum catalysts are crucial in the process that converts naphtha into petrol, diesel, and jet-engine fuel, which are all vital to the global economy. The emissions from those petroleum fuels, however, can be toxic, and platinum is also crucial in the worldwide push to reduce them through automotive catalytic converters. In fact, 2% of global platinum use in 2016 was in converting petroleum and 41% went into reducing emissions – a circle of platinum use that’s more impressive than a ring.
2. Feed the world

Nitric acid is a by-product of platinum which is used in fertilisers. Image: Pixabay
Another vital global sector that makes use of platinum catalysts is agriculture. Without synthetic fertilisers, we would not be able to produce nearly as much food as we need. Nitric acid is essential for producing those fertilisers and platinum is essential for producing nitric acid. Since 90% of the gauzes required for nitric acid are platinum, we may need to use more of it as we try to meet the global food challenge.
3. Good for your health

A pacemaker. Image: Steven Fruitsmaak@Wikimedia Commons
Platinum is extremely hard wearing, non-corrosive, and highly biocompatible, making it an excellent material to protect medical implants from acid corrosion in the human body. It is commonly used in pacemakers and stents. It is also used in chemotherapy, where platinum-based chemotherapeutic agents are used to treat up to 50% of cancer patients.
Keep reading

The dihydrogen monoxide hoax involves calling water by the unfamiliar chemical name “dihydrogen monoxide” (DHMO), and listing some of water’s effects in an alarming manner, such as the fact that it accelerates corrosion and can cause severe burns. The hoax often calls for dihydrogen monoxide to be regulated, labeled as hazardous, or banned. It illustrates how the lack of scientific literacy and an exaggerated analysis can lead to misplaced fears.
The hoax gained renewed popularity in the late 1990s when a 14-year-old student collected anti-DHMO petitions for a science project about gullibility. The story has since been used in science education to encourage critical thinking and avoid the appeal to nature.
Forty-three students favored banning DHMO, six were undecided, and only one correctly recognized that ‘dihydrogen monoxide’ is actually plain old water.
Here’s the information he gave the students:
Dihydrogen monoxide is colorless, odorless, tasteless, and kills uncounted thousands of people every year. Most of these deaths are caused by accidental inhalation of DHMO, but the dangers of dihydrogen monoxide do not end there. Prolonged exposure to its solid form causes severe tissue damage. Symptoms of DHMO ingestion can include excessive sweating and urination, and possibly a bloated feeling, nausea, vomiting and body electrolyte imbalance. For those who have become dependent, DHMO withdrawal means certain death.
Dihydrogen monoxide:
is also known as hydroxl acid, and is the major component of acid rain.
contributes to the “greenhouse effect.”
may cause severe burns.
contributes to the erosion of our natural landscape.
accelerates corrosion and rusting of many metals.
may cause electrical failures and decreased effectiveness of automobile brakes.
has been found in excised tumors of terminal cancer patients.
Contamination is reaching epidemic proportions!
Quantities of dihydrogen monoxide have been found in almost every stream, lake, and reservoir in America today. But the pollution is global, and the contaminant has even been found in Antarctic ice. DHMO has caused millions of dollars of property damage in the midwest, and recently California.
Despite the danger, dihydrogen monoxide is often used:
as an industrial solvent and coolant.
in nuclear power plants.
in the production of styrofoam.
as a fire retardant.
in many forms of cruel animal research.
in the distribution of pesticides. Even after washing, produce remains contaminated by this chemical.
as an additive in certain “junk-foods” and other food products.
Companies dump waste DHMO into rivers and the ocean, and nothing can be done to stop them because this practice is still legal. The impact on wildlife is extreme, and we cannot afford to ignore it any longer!
The American government has refused to ban the production, distribution, or use of this damaging chemical due to its “importance to the economic health of this nation.” In fact, the navy and other military organizations are conducting experiments with DHMO, and designing multi-billion dollar devices to control and utilize it during warfare situations. Hundreds of military research facilities receive tons of it through a highly sophisticated underground distribution network. Many store large quantities for later use.
Source: [x]
Click HERE for more facts
-
 phoniexs-world liked this · 4 months ago
phoniexs-world liked this · 4 months ago -
 harlleyqqueen liked this · 1 year ago
harlleyqqueen liked this · 1 year ago -
 jaicourtneyfan liked this · 1 year ago
jaicourtneyfan liked this · 1 year ago -
 benbemine liked this · 1 year ago
benbemine liked this · 1 year ago -
 rekles liked this · 2 years ago
rekles liked this · 2 years ago -
 givemebaconoryoudie liked this · 3 years ago
givemebaconoryoudie liked this · 3 years ago -
 bionicle51566 liked this · 3 years ago
bionicle51566 liked this · 3 years ago -
 monaisme liked this · 3 years ago
monaisme liked this · 3 years ago -
 monaisme reblogged this · 3 years ago
monaisme reblogged this · 3 years ago -
 bernard-robert reblogged this · 3 years ago
bernard-robert reblogged this · 3 years ago -
 tgfangirl4eva liked this · 3 years ago
tgfangirl4eva liked this · 3 years ago -
 leftovercheesecake liked this · 3 years ago
leftovercheesecake liked this · 3 years ago -
 marvellousidiot liked this · 4 years ago
marvellousidiot liked this · 4 years ago -
 eliza1911o1 liked this · 4 years ago
eliza1911o1 liked this · 4 years ago -
 lijabook liked this · 4 years ago
lijabook liked this · 4 years ago -
 whamsworld liked this · 4 years ago
whamsworld liked this · 4 years ago -
 insanityreignsoveroblivion liked this · 4 years ago
insanityreignsoveroblivion liked this · 4 years ago -
 miscourage liked this · 4 years ago
miscourage liked this · 4 years ago -
 spiderdetentionaire liked this · 4 years ago
spiderdetentionaire liked this · 4 years ago -
 lightoftheseraph liked this · 4 years ago
lightoftheseraph liked this · 4 years ago -
 samaras-weavings liked this · 4 years ago
samaras-weavings liked this · 4 years ago -
 marvel-iron-man reblogged this · 4 years ago
marvel-iron-man reblogged this · 4 years ago -
 dreaming-in-the-dark reblogged this · 4 years ago
dreaming-in-the-dark reblogged this · 4 years ago -
 dreaming-in-the-dark liked this · 4 years ago
dreaming-in-the-dark liked this · 4 years ago -
 sielutonlampikana liked this · 4 years ago
sielutonlampikana liked this · 4 years ago -
 inkymooniko liked this · 4 years ago
inkymooniko liked this · 4 years ago -
 killjoytoxicberry liked this · 4 years ago
killjoytoxicberry liked this · 4 years ago -
 winchsterzs reblogged this · 4 years ago
winchsterzs reblogged this · 4 years ago -
 sendevereaux liked this · 4 years ago
sendevereaux liked this · 4 years ago -
 darkflamethedragondame liked this · 4 years ago
darkflamethedragondame liked this · 4 years ago -
 keywolf13 liked this · 4 years ago
keywolf13 liked this · 4 years ago -
 hero-in-high-tops liked this · 4 years ago
hero-in-high-tops liked this · 4 years ago -
 24601error-prisonernotfound reblogged this · 4 years ago
24601error-prisonernotfound reblogged this · 4 years ago -
 eveoftheleaf liked this · 4 years ago
eveoftheleaf liked this · 4 years ago -
 superblawyerclamhumanoid liked this · 4 years ago
superblawyerclamhumanoid liked this · 4 years ago -
 memelatonin liked this · 5 years ago
memelatonin liked this · 5 years ago -
 l-egionaire liked this · 5 years ago
l-egionaire liked this · 5 years ago -
 batangkilljoy liked this · 5 years ago
batangkilljoy liked this · 5 years ago -
 m0wavy liked this · 5 years ago
m0wavy liked this · 5 years ago -
 voyeurysm liked this · 5 years ago
voyeurysm liked this · 5 years ago -
 ironcible liked this · 5 years ago
ironcible liked this · 5 years ago -
 thevoidofspaceandtime liked this · 5 years ago
thevoidofspaceandtime liked this · 5 years ago -
 zselenophile reblogged this · 5 years ago
zselenophile reblogged this · 5 years ago -
 zselenophile liked this · 5 years ago
zselenophile liked this · 5 years ago -
 that-spider-fan-over-there liked this · 5 years ago
that-spider-fan-over-there liked this · 5 years ago