Amateurchemstudent - Untitled

More Posts from Amateurchemstudent and Others
Polarity, Resonance, and Electron Pushing: Crash Course Organic Chemistry #10:
We’ve all heard the phrase “opposites attract.” It may or may not be true for people, but it’s definitely true in organic chemistry. In this episode of Crash Course Organic Chemistry, we’re learning about electronegativity, polarity, resonance structures, and resonance hybrids. We’ll practice a very important skill for this course that will help us avoid a lot of memorization in the future: electron pushing. It’ll be a lot of trial and error at first, but we all start somewhere!
What comes to mind when you think of alcohol? Probably alcoholic drinks like beer or wine. But in organic chemistry alcohols are an important and versatile family of compounds. In this episode of Crash Course Organic Chemistry, we’ll use alcohols as a starting point to get other types of compounds like ethers, epoxides, and more!
It’s World Sleep Day
Log off.
Go back to bed.
Water: Making a Splash
You don’t have to be a genius to know that water is essential for life. After all, we’re made up of it, we sweat it, we drink it, some people even opt to give birth in it. But what is it about two hydrogens and an oxygen which make it so sensational?
The answer is to do with water’s structure. A H2O molecule is covalently bonded, which means each atom shares electrons. In this case, the covalent bonds are between two hydrogen atoms and one oxygen atom. Oxygen is cool because it is highly electronegative. Electronegativity is the ability for one atom to “pull” the electrons towards it in a covalent bond. Since oxygen is highly electronegative, it pulls the electrons in the bond towards it which gives the oxygen a slight negative charge because of the electron proximity. This is represented by δ- (delta negative). The hydrogen is therefore δ+ (delta positive) and has a slight positive charge. Overall, the molecule is said to be polar, or to be dipolar in nature, because there is a difference in charge across the molecule.
Water being a dipole gives it different properties, which you need to know about if you are sitting the AS or A level biology exam.
A quick note on hydrogen bonding…
Being a dipole, water has areas of different charge. When many molecules come together, hydrogen bonds can form between H+ on one molecule and O- on another, shown in the diagram with a dashed line.

It is hydrogen bonds which give water a property called surface tension. Water has a high tendency to ‘stick together’, called cohesion. This is important in water transport through the xylem in later units. Surface tension is a bit like a “skin” because it can allow small organisms to walk along it. It occurs because water molecules on the surface bond to their neighbours much like throughout the whole liquid, but since one side is exposed to air and cannot form hydrogen bonds upwards, they will form stronger ones with the molecules beside them. The net attraction is downwards.
Water is good as a temperature buffer too. Heating a substance makes its particles gain more kinetic energy and therefore the overall temperature rises since particles are moving faster. With water, the temperature doesn’t rise as much as other liquids do. This is because it takes more heat energy to raise the temperature of water by 1 degree - it has a high specific heat capacity due to the many hydrogen bonds that have to be broken (even though they are weak on their own). It takes a lot of heat energy for water to raise its temperature significantly.
This is useful in organisms because our cells are mostly water, which can absorb heat energy without raising our temperature very much. Therefore it “buffers” or reduces heat changes. Seas, lakes and oceans are all good environments to live in because they do not change temperature as quickly as air. Aquatic organisms have an environment with less temperature fluctuation than land organisms.
Having a high latent heat of vaporisation means water can cool down organisms by evaporating a small amount of water. Evaporation is when water becomes a gas due to the large amount of KE. Fast-moving molecules are removed when this occurs and take their energy with them, therefore decreasing the amount of energy left behind and cooling it. Sweat is a good example of high latent heat of vaporisation. A small quantity of water is removed with a large cooling effect, meaning temperature is stabilised without losing a lot of water.
Water is also a good solvent (a substance which can dissolve other substances) and this is due to more hydrogen bonding. Water’s charges of H+ and O- are attracted to the positive and negative charges on molecules and therefore solutes such as NaCl are split into Na+ and Cl-, then spread out. Solvent properties are important in transport (such as blood plasma dissolving glucose, vitamins, urea etc), metabolic reactions, urine production and mineral transportation through the xylem and phloem in plants.
Water molecules can also take place in metabolic reactions. Hydrolysis reactions involve breaking down the covalent bonds between hydrogen and oxygen and making new ones, for example, in digestion. Condensation reactions produce water as a byproduct e.g. the formation of phosphodiester bonds. Water is referred to as a metabolite.
Summary
Water is a dipole due to the slight opposite charges on oxygen and hydrogen atoms.
Hydrogen bonds form between hydrogens on one water molecule and oxygens on another.
Because of this, water has the tendency to stick to itself - cohesion. Cohesion is the reason for surface tension.
Water is a good temperature buffer because of its high specific heat capacity. It takes a lot of energy to raise the temperature by a degree.
Water has a high latent heat of vaporisation so evaporating a little has a large cooling effect.
Water is a good solvent because of how the hydrogen bonds attract charged molecules and separate them. This is useful for transporting solutions.
Water is a metabolite important for hydrolysis reactions and produced in condensation reactions.
Happy studying!
Covalent Bonds: Sharing Is Caring!
Welcome to my second out of three posts on bonding - ionic, covalent and metallic. This post also covers the coordinate/ dative bond which I can’t remember if I’ve covered before. Only one more of this series left! Find the others here.
Covalent bonding involves one or more shared pairs of electrons between two atoms. These can be found in simple molecular elements and compounds like CO2 , macromolecular structures like diamond and molecular ions such as ammonium. Covalent bonds mostly occur between non-metals but sometimes metals can form covalent bonds.
Single covalent bonds share just one pair of electrons. Double covalent bonds share two. Triple covalent bonds share three.
Each atom usually provides one electron – unpaired in the orbital – in the bond. The number of unpaired electrons in an atom usually shows how many bonds it can make but sometimes atoms promote electrons to fit in more. Covalent bonds are represented with lines between the atoms – double and triple bonds represented with two and three lines respectively.
Dot and cross diagrams show the arrangement of electrons in covalent bonds. They use dots and crosses to demonstrate that the electrons come from different places and often only the outer shell is shown.

The simple explanation as to how atoms form covalent bonds is that one unpaired electron in the orbital of one atom overlaps with one in another atom. Sometimes atoms promote electrons in the same energy level to form more covalent bonds. For example, if an atom wants to make three covalent bonds but has a full 3s2 shell and a 3p1 shell, it can promote one of its 3s2 electrons so that an electron from the other atoms can fill the 3s shell and pair with the new 3p2 shell.
Sometimes promotion does not occur and that means different compounds can be made such as PCl3 or PCl5.

A lone pair of electrons is a pair of electrons from the same energy sub-level uninvolved in bonding. Sometimes these can form something called a coordinate bond, which contains a shared pair of electrons where both come from one atom. The lone pair of electrons is “donated” into the empty orbital of another atom to form a coordinate bond.

This is an example of a coordinate (sometimes called dative) bond between ammonia and a H+ ion which has an empty orbital. The lone pair on the ammonia overlaps with this H+ ion and donates its electrons. Both electrons come from the ammonia’s lone pair so it is a coordinate bond. This is demonstrated with an arrow. The diagram is missing an overall charge of + on the ammonium ion it produces. Coordinate bonds act the same as covalent bonds.
Once you have your covalent bonds, you need to know about covalent substances and their properties. There are two types of covalent substance: simple covalent (molecular) and macromolecular (giant covalent).
Molecular simply means that the formula for the compound or element describes exactly how many atoms are in one molecule, e.g. H2O. Molecular covalent crystalline substances usually exist as single molecules such as iodine or oxygen. They are usually gases or liquids at room temperature but can be low melting point solids.
Solid molecular covalent solids are crystalline so can be called molecular covalent crystals. Iodine and ice are examples of these. Iodine (shown below) has a regular arrangement which makes it a crystalline substance and water, as ice, has a crystalline structure as well.

The properties of these crystals are that they have low melting points, are very brittle due to the lack of strong bonds holding them together and also do not conduct electricity since no ions are present.
The other kind of covalent substance you need to know is macromolecular. This includes giant covalent structures such as diamond or graphite, which are allotropes of carbon. Non-metallic elements and compounds usually form these crystalline structures with a regular arrangement of atoms.
Allotropes are different forms of the same element in the same physical state.
Diamond is the hardest naturally occurring substance on earth therefore is good for cutting glass and drilling and mining. It has a high melting point due to the many covalent bonds which require a lot of energy to break. Each carbon has four of these bonds joining it to four others in a tetrahedral arrangement with a bond angle of 109.5 degrees and it does not conduct electricity or heat because there are no ions free to move.
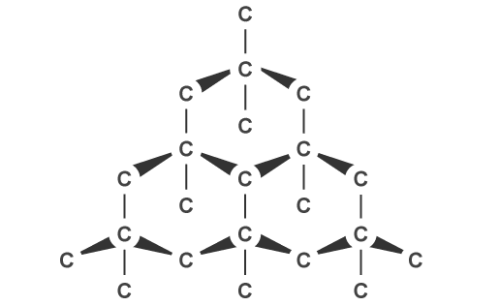
Graphite, on the other hand, can conduct electricity. This is because it has delocalised electrons between the layers which move and carry charge. Carbon atoms within the structure are only bonded to three others in a hexagonal arrangement with a bond angle of 120 degrees. Since only three of carbon’s unpaired electrons are used in bonding, the fourth becomes delocalised and moves between the layers of graphite causing weak attractions, explaining why it can conduct electricity.

Graphite’s layered structure and the weak forces of attractions between it make it a good lubricant and ideal for pencil lead because the layers can slide over each other. The attractions can be broken easily but the covalent bonds within the layers give graphite a high melting point due to the amount of energy needed to break them.
SUMMARY
Covalent bonding involves one or more shared pairs of electrons between two atoms. Covalent bonds mostly occur between non-metals but sometimes metals can form covalent bonds.
Single covalent bonds share just one pair of electrons. Double covalent bonds share two. Triple covalent bonds share three.
Each atom usually provides one electron – unpaired in the orbital – in the bond. The number of unpaired electrons in an atom usually shows how many bonds it can make but sometimes atoms promote electrons to fit in more. Covalent bonds are represented with lines between the atoms.
Dot and cross diagrams use dots and crosses to demonstrate that the electrons come from different places and often only the outer shell is shown.
The simple explanation as to how atoms form covalent bonds is that one unpaired electron in the orbital of one atom overlaps with one in another atom. Sometimes atoms promote electrons in the same energy level to form more covalent bonds.
Sometimes promotion does not occur and that means different compounds can be made such as PCl3 or PCl5.
A lone pair of electrons is a pair of electrons from the same energy sub-level uninvolved in bonding. Sometimes these can form something called a coordinate bond, which contains a shared pair of electrons where both come from one atom. The lone pair of electrons is “donated” into the empty orbital of another atom to form a coordinate bond.
The formation of ammonium is an example of this.
There are two types of covalent substance: simple covalent (molecular) and macromolecular (giant covalent).
Molecular simply means that the formula for the compound or element describes exactly how many atoms are in one molecule, e.g. H2O. Molecular covalent crystalline substances usually exist as single molecules such as iodine or oxygen. They are usually gases or liquids at room temperature but can be low melting point solids.
Solid molecular covalent solids are crystalline so can be called molecular covalent crystals. Iodine and ice are examples of these.
The properties of these crystals are that they have low melting points, are very brittle due to the lack of strong bonds holding them together and also do not conduct electricity since no ions are present.
Giant covalent structures such as diamond or graphite are allotropes of carbon. Allotropes are different forms of the same element in the same physical state.
Diamond has a high melting point due to the many covalent bonds which require a lot of energy to break. Each carbon has four of these bonds joining it to four others in a tetrahedral arrangement with a bond angle of 109.5 degrees and it does not conduct electricity or heat because there are no ions free to move.
Graphite can conduct electricity. This is because it has delocalised electrons between the layers which move and carry charge. Carbon atoms within the structure are only bonded to three others in a hexagonal arrangement with a bond angle of 120 degrees. Since only three of carbon’s unpaired electrons are used in bonding, the fourth becomes delocalised and moves between the layers of graphite causing weak attractions, explaining why it can conduct electricity.
Graphite’s layered structure and the weak forces of attractions between it make it a good lubricant and ideal for pencil lead because the layers can slide over each other. The attractions can be broken easily but the covalent bonds within the layers give graphite a high melting point due to the amount of energy needed to break them.
Happy studying!
The Name’s Bond ... Ionic Bond.
This is the first in my short series of the three main types of bond - ionic, metallic and covalent. In this, you’ll learn about the properties of the compounds, which atoms they’re found between and how the bonds are formed. Enjoy!
When electrons are transferred from a metal to a non-metal, an ionic compound is formed. Metals usually lose electrons and non-metals usually gain them to get to a noble gas configuration. Transition metals do not always achieve this.
Charged particles that have either lost or gained electrons are called ions and are no longer neutral - metal atoms lose electrons to become positive ions (cations) whereas non-metals gain electrons to become negative ions (anions).
The formation of these ions is usually shown using electron configurations. Make sure you know that the transfer of electrons is not the bond but how the ions are formed.
An ionic bond is the electrostatic attraction between oppositely charged ions.
You need to know how to explain how atoms react with other atoms and for this the electron configurations are needed. You can use dot and cross diagrams for this.

Ionic solids hold ions in 3D structures called ionic lattices. A lattice is a repeating 3D pattern in a crystalline solid. For example, NaCl has a 6:6 arrangement - each Na+ ion is surrounded by 6 Cl- and vice versa.
Ionic solids have many strong electrostatic attractions between their ions. The crystalline shape can be decrepitated (cracked) on heating. Ionic Lattices have high melting and boiling points since they need more energy to break because atoms are held together by lots of strong electrostatic attractions between positive and negative ions. The boiling point of an ionic compound depends on the size of the atomic radius and the charge of the ion. The smaller the ion and the higher the charge, the stronger attraction.

Look at this diagram. It shows how atomic radius decreases across a period regularly. This is not the case with the ions. Positive ions are usually smaller than the atoms they came from because metal atoms lose electrons meaning the nuclear charge increases which draws the electrons closer to the nucleus. For negative ions, they become larger because repulsion between electrons moves them further away - nuclear charge also decreases as more electrons to the same number of protons.
Ionic substances can conduct electricity through the movement of charged particles when molten or dissolved (aqueous). This is because when they are like this, electrons are free to move and carry a charge. Ionic solids cannot conduct electricity.

Ionic compounds are usually soluble in water. This is because the polar water molecules cluster around ions which have broken off the lattice and so separate them from each other. Some substances like aluminium oxide have too strong electrostatic attractions so water cannot break up the lattice - it is insoluble in water.
Molecular ions such as sulfate, nitrate, ammonium or carbonate can exist within ionic compounds. These compounds may have covalent bonds within the ions but overall they are ionic and exhibit thee properties described above.
SUMMARY
When electrons are transferred from a metal to a non-metal, an ionic compound is formed.
Charged particles that have either lost or gained electrons are called ions and are no longer neutral - metal atoms lose electrons to become positive ions (cations) whereas non-metals gain electrons to become negative ions (anions).
The formation of these ions is usually shown using electron configurations. The transfer of electrons is not the bond but how the ions are formed.
An ionic bond is the electrostatic attraction between oppositely charged ions.
Ionic solids hold ions in 3D structures called ionic lattices. A lattice is a repeating 3D pattern in a crystalline solid.
Ionic solids have many strong electrostatic attractions between their ions. The crystalline shape can be decrepitated (cracked) on heating.
Ionic Lattices have high melting and boiling points since they need more energy to break because atoms are held together by lots of strong electrostatic attractions between positive and negative ions.
The boiling point of an ionic compound depends on the size of the atomic radius and the charge of the ion. The smaller the ion and the higher the charge, the stronger attraction.
Positive ions are usually smaller than the atoms they came from because metal atoms lose electrons meaning the nuclear charge increases which draws the electrons closer to the nucleus. Negative ions become larger because repulsion between electrons moves them further away - nuclear charge also decreases as more electrons to the same number of protons.
Ionic substances can conduct electricity through the movement of charged particles when molten or dissolved (aqueous). This is because when they are like this, electrons are free to move and carry a charge. Ionic solids cannot conduct electricity.
Ionic compounds are usually soluble in water because the polar water molecules cluster around ions which have broken off the lattice and so separate them from each other.
Some substances like aluminium oxide have too strong electrostatic attractions so water cannot break up the lattice - it is insoluble in water.
Molecular ions such as sulfate, nitrate, ammonium or carbonate can exist within ionic compounds. These compounds may have covalent bonds within the ions but overall they are ionic and exhibit thee properties described above.

Alkanes: Crash Course Organic Chemistry #6:
Alkanes are kind of the wallflowers of organic chemistry, but they still have important functions in the world around us. In this episode of Crash Course Organic Chemistry we’re building our knowledge of organic molecules by learning all about these so called couch potatoes from how they are separated from crude oil to how to use Newman projections to predict torsional strain and steric hinderance. We’ll also learn the names of some common conformers and get an introduction to cycloalkanes.
-
 choosing-hope liked this · 3 years ago
choosing-hope liked this · 3 years ago -
 tiburonc1n reblogged this · 3 years ago
tiburonc1n reblogged this · 3 years ago -
 tiburonc1n liked this · 3 years ago
tiburonc1n liked this · 3 years ago -
 lawyalltea reblogged this · 4 years ago
lawyalltea reblogged this · 4 years ago -
 potatobi reblogged this · 4 years ago
potatobi reblogged this · 4 years ago -
 appasinvisiblefriend liked this · 4 years ago
appasinvisiblefriend liked this · 4 years ago -
 aniihera liked this · 4 years ago
aniihera liked this · 4 years ago -
 threeofswordsx liked this · 4 years ago
threeofswordsx liked this · 4 years ago -
 lady-bluebird reblogged this · 4 years ago
lady-bluebird reblogged this · 4 years ago -
 lady-bluebird liked this · 4 years ago
lady-bluebird liked this · 4 years ago -
 infinitely-caffeinated liked this · 4 years ago
infinitely-caffeinated liked this · 4 years ago -
 sterekandlarry reblogged this · 4 years ago
sterekandlarry reblogged this · 4 years ago -
 sterekandlarry liked this · 4 years ago
sterekandlarry liked this · 4 years ago -
 firlachiel reblogged this · 4 years ago
firlachiel reblogged this · 4 years ago -
 ladymeep reblogged this · 4 years ago
ladymeep reblogged this · 4 years ago -
 nerd-on-duty reblogged this · 4 years ago
nerd-on-duty reblogged this · 4 years ago -
 tt28 liked this · 4 years ago
tt28 liked this · 4 years ago -
 jams-jellies liked this · 4 years ago
jams-jellies liked this · 4 years ago -
 bananananice liked this · 4 years ago
bananananice liked this · 4 years ago -
 nerd-on-duty liked this · 4 years ago
nerd-on-duty liked this · 4 years ago -
 jonairadreaming liked this · 4 years ago
jonairadreaming liked this · 4 years ago -
 tigerliliesandcherryblossoms reblogged this · 4 years ago
tigerliliesandcherryblossoms reblogged this · 4 years ago -
 tigerliliesandcherryblossoms liked this · 4 years ago
tigerliliesandcherryblossoms liked this · 4 years ago -
 yourusernameiswhat liked this · 4 years ago
yourusernameiswhat liked this · 4 years ago -
 thatspookyagent reblogged this · 4 years ago
thatspookyagent reblogged this · 4 years ago -
 momomun liked this · 4 years ago
momomun liked this · 4 years ago -
 confidantjustice liked this · 4 years ago
confidantjustice liked this · 4 years ago -
 skorpyius liked this · 4 years ago
skorpyius liked this · 4 years ago -
 hes-a-merman liked this · 4 years ago
hes-a-merman liked this · 4 years ago -
 emiliazebra liked this · 4 years ago
emiliazebra liked this · 4 years ago -
 the-dirty-cowboy liked this · 4 years ago
the-dirty-cowboy liked this · 4 years ago -
 gambitsobsession reblogged this · 4 years ago
gambitsobsession reblogged this · 4 years ago -
 easily-broken-by-emotion reblogged this · 4 years ago
easily-broken-by-emotion reblogged this · 4 years ago -
 easily-broken-by-emotion liked this · 4 years ago
easily-broken-by-emotion liked this · 4 years ago -
 herequeeranduncomfortable reblogged this · 4 years ago
herequeeranduncomfortable reblogged this · 4 years ago -
 13marvelfan13 liked this · 4 years ago
13marvelfan13 liked this · 4 years ago -
 satans-my-apprentice reblogged this · 4 years ago
satans-my-apprentice reblogged this · 4 years ago -
 phlebasphoenician liked this · 4 years ago
phlebasphoenician liked this · 4 years ago -
 laughinguntilmysidehurts reblogged this · 4 years ago
laughinguntilmysidehurts reblogged this · 4 years ago -
 lucasscheijdephoto liked this · 4 years ago
lucasscheijdephoto liked this · 4 years ago -
 just-a-waifu-lover liked this · 4 years ago
just-a-waifu-lover liked this · 4 years ago -
 the-name-i-never-wanted liked this · 4 years ago
the-name-i-never-wanted liked this · 4 years ago -
 notverysmart liked this · 4 years ago
notverysmart liked this · 4 years ago -
 blahwesome liked this · 4 years ago
blahwesome liked this · 4 years ago -
 jinxkitt reblogged this · 4 years ago
jinxkitt reblogged this · 4 years ago -
 brainfrogtraveller liked this · 4 years ago
brainfrogtraveller liked this · 4 years ago -
 stampothy reblogged this · 4 years ago
stampothy reblogged this · 4 years ago -
 meigalaxy liked this · 4 years ago
meigalaxy liked this · 4 years ago

