Happy #StPatricksDay2021! Ever Wondered Why The Bubbles In Guinness Appear To Fall Rather Than Rise,

Happy #StPatricksDay2021! Ever wondered why the bubbles in Guinness appear to fall rather than rise, and what causes its dark colour? This graphic in C&EN has the answers! https://ift.tt/2WfNI8j https://ift.tt/3rY7DIW
More Posts from Amateurchemstudent and Others
It’s World Sleep Day
Log off.
Go back to bed.
Haloalkanes and Their Angelic Reactions: Part One
Haloalkanes are more commonly referred to as halogenoalkanes. Obviously you’ve already read my post on halogenoalkanes and their properties so there’s no surprise that you’re itching to read what I’ve got to say about these beauties and their reactions! Should we delve in?
There are a few different kinds of reactions you must learn for the A Level exam that involve halogenoalkanes.
The first is the synthesis of chloroalkanes via the photochemical chlorination of the alkanes. I know it looks scary, but don’t worry, it is simpler than it sounds. It essentially means “forming chloroalkanes through chlorinating an alkane in the presence of sunlight”.
Chlorine will react with methane when UV light is present and will form several kinds of chloroalkanes and fumes of hydrogen chloride gas. Chloromethane was once commonly used as a refridgerant. Depending on how many chlorine molecules there are, there will be different compounds formed:
methane + chlorine -> chloromethane + hydrogen chloride
CH4 + Cl2 -> CH3Cl + HCl
or
methane + chlorine -> trichloromethane + hydrogen chloride
CH4 + 3Cl2 -> CHCl3 + 3HCl
When undergone in real life, mixtures of halogenoalkanes are produced with some long chain alkanes which can be separated out with fractional distillation.
To understand what happens in an overall chemical reaction, chemists use mechanisms. These basically show the step-by-step process that is usually shown by a simple symbol equation that summarises everything.
The chlorination of methane is something you must learn the mechanism for. It’s pretty easy but involves a lot of steps and must be revised periodically to remember them.
The actual reaction is a substitution reaction because one atom or group is replaced by another. Since the chlorine involved is a free radical, it can also be called a free-radical substitution reaction.
1. Initiation
UV light is essential for the first step in the mechanism. This breaks the Cl-Cl covalent bond so that each chlorine leaves with one electron from the shared pair. Chlorine free radicals, with one unpaired electron in the outer shell, are formed. Free radicals are only formed if a bond splits evenly - each atom getting one of the two electrons. The name given to this is homolytic fission.

2. Propagation
This has two sub-steps
(a) Chlorine free radicals (highly reactive) react with methane to form hydrogen chloride and leave a methyl free radical.
Cl• + CH4 -> HCl + •CH3
(b) This free radical then reacts with another chlorine to form chloromethane and another chlorine free radical. Producing free radicals is a chain reaction which is why it is such a problem in ozone depletion - a little amount can cause a lot of destruction.
•CH3 + Cl2 -> CH3Cl + •Cl
3. Termination
This step stops the chain reaction. It only happens when two free radicals collide to form a molecule in several ways:
Cl• + Cl• -> Cl2
UV light would just break down the chlorine molecule again, so although this is technically a termination reaction it is not the most efficient.
Cl• + •CH3 -> CH3Cl
Forming one molecule of methane uses one chlorine and one methyl free radical.
•CH3 + •CH3 -> C2H6
Ethane can be formed from two methyl free radicals - this is why there are longer chain alkanes in the mixture.
This whole process is how organic halogenoalkanes are the product of photochemical reactions of halogens with alkanes in UV light - made via free radical substitution mechanisms in chain reaction.
Another reaction you need to know is a nucleophilic substitution reactions. A nucleophile is an electron pair donor or proton acceptor - the name comes from Greek origins (”loves nucleus”) - such as hydroxide ions, cyanide ions or ammonia molecules. Hydroxide and cyanide ions are negative but ammonia is neutral.

Halogenoalkanes have a polar bond because of the difference between the highly electronegative halogen and the carbon atom. The 𝛿+ carbon can go under nucleophilic attack. The mechanism for negatively charged nucleophiles these in general is:

Nu represents the nucleophile. This example is with a bromoalkane. Make sure to include curly arrows that begin at a lone pair or the centre of a bond and end at an atom or centre of bond, and delta (slight) charges.
Lets look at a more specific example:
One nucleophile that can be used is a hydroxide ion, found in either water or sodium hydroxide. In this case, you need to know about aqueous sodium hydroxide or potassium hydroxide and a halogenoalkane. This takes place at room temperature but is slow so is often refluxed (continuously boiled and condensed back into the reaction flask). Reflux apparatus is shown below:

The halogenoalkane is dissolved into ethanol since it is insoluable in water and this solution along with the aqueous hydroxide can mix. The product produced is an alcohol, which is organic.
The general reaction is:
R-CH2X + NaOH -> CH3CH2OH + NaX
Where X represents a halogen.
You must learn the mechanism for this reaction. The lone pair on the hydroxide attacks the carbon atom attached to the halogen and this causes both carbon electrons to move to the halogen which becomes a halide ion.

The reaction of a hydroxide ion can also be classed as a hydrolysis reaction as it breaks down chemical bonds with water or hydroxide ions. The speed of reaction depends on the strength of the bond - a stronger carbon-halogen bond, a slower reaction.

C-I is the most reactive (reactivity increases down group 7) and C-F is therefore the least reactive and strongest.
Part two of this post will cover nucleophilic substitution of cyanide ions and ammonia molecules, as well as elimination reactions.
SUMMARY
You need to know about the synthesis of chloroalkanes via the photochemical chlorination of the alkanes. - “forming chloroalkanes through chlorinating an alkane in the presence of sunlight”.
Chlorine will react with methane when UV light is present and will form several kinds of chloroalkanes and fumes of hydrogen chloride gas. Depending on how many chlorine molecules there are, there will be different compounds formed.
When undergone in real life, mixtures of halogenoalkanes are produced with some long chain alkanes which can be separated out with fractional distillation.
To understand what happens in an overall chemical reaction, chemists use mechanisms. These basically show the step-by-step process.
The chlorination of methane is something you must learn the mechanism for. The actual reaction is a substitution reaction because one atom or group is replaced by another.
The first step is initiation - UV light is essential for the first step in the mechanism. This breaks the Cl-Cl covalent bond so that each chlorine leaves with one electron from the shared pair. Chlorine free radicals, with one unpaired electron in the outer shell, are formed. Free radicals are only formed if a bond splits evenly - each atom getting one of the two electrons.
Step two is propagation: (a) Chlorine free radicals (highly reactive) react with methane to form hydrogen chloride and leave a methyl free radical (b) this free radical then reacts with another chlorine to form chloromethane and another chlorine free radical. Producing free radicals is a chain reaction which is why it is such a problem in ozone depletion - a little amount can cause a lot of destruction.
To stop the chain reaction, the final step is termination. It only happens when two free radicals collide to form a molecule in several ways: two chlorine free radicals forming a chlorine molecule, two methyl FRs forming ethane or a chlorine FR and a methyl FR forming chloromethane.
Ethane contributes to the longer chain alkanes in the mixture.
Another reaction you need to know is a nucleophilic substitution reactions. A nucleophile is an electron pair donor or proton acceptor, such as hydroxide ions, cyanide ions or ammonia molecules. Hydroxide and cyanide ions are negative but ammonia is neutral.
Halogenoalkanes have a polar bond because of the difference between the highly electronegative halogen and the carbon atom. The 𝛿+ carbon can go under nucleophilic attack.
Nu represents the nucleophile. Make sure to include curly arrows that begin at a lone pair or the centre of a bond and end at an atom or centre of bond, and delta (slight) charges.
One nucleophile that can be used is a hydroxide ion, found in either water or sodium hydroxide. In this case, you need to know about aqueous sodium hydroxide or potassium hydroxide and a halogenoalkane. This takes place at room temperature but is slow so is often refluxed (continuously boiled and condensed back into the reaction flask). The halogenoalkane is dissolved into ethanol since it is insoluable in water and this solution along with the aqueous hydroxide can mix. The product produced is an alcohol, which is organic.
The general reaction is :R-CH2X + NaOH -> CH3CH2OH + NaX where X represents a halogen
The lone pair on the hydroxide attacks the carbon atom attached to the halogen and this causes both carbon electrons to move to the halogen which becomes a halide ion.
The reaction of a hydroxide ion can also be classed as a hydrolysis reaction as it breaks down chemical bonds with water or hydroxide ions.
The speed of reaction depends on the strength of the bond - a stronger carbon-halogen bond, a slower reaction. C-I is the most reactive (reactivity increases down group 7) and C-F is therefore the least reactive and strongest.
Combusting Alkanes
If you follow this blog, by now you must be thinking, when will we be done with the alkane chemistry? Well, the answer is never. There is still one more topic to touch on - burning alkanes and the environmental effects. Study up chums!
Alkanes are used as fuels due to how they can combust easily to release large amounts of heat energy. Combustion is essentially burning something in the presence of oxygen. There are two types of combustion: complete and incomplete.
Complete combustion occurs when there is a plentiful supply of air. When an alkane is burned in sufficient oxygen, it produces carbon dioxide and water. How much depends on what is being burnt. For example:
butane + oxygen -> carbon dioxide + water
2C4H10 (g) + 13O2 (g) -> 8CO2 (g) + 10H2O (g)
Remember state symbols in combustion reactions. In addition, this reaction can be halved to balance for 1 mole of butane by using fractions when dealing with the numbers.
C4H10 (g) + 6 ½ O2 (g) -> 4CO2 (g) + 5H2O (g)
Incomplete combustion on the other hand occurs when there is a limited supply of air. There are two kinds of incomplete combustion. The first type produces water and carbon monoxide.
butane + limited oxygen -> carbon monoxide + water
C4H10 (g) + 4 ½ O2 (g) -> 4CO (g) + 5H2O (g)
Carbon monoxide is dangerous because it is toxic and undetectable due to being smell-free and colourless. It reacts with haemoglobin in your blood to reduce their oxygen-carrying ability and can cause drowsiness, nausea, respiratory failure or death. Applicances therefore must be maintained to prevent the formation of the monoxide.
The other kind of incomplete combustion occurs in even less oxygen. It produces water and soot (carbon).
butane + very limited oxygen -> carbon + water
C4H10 (g) + 2 ½ O2 (g) -> 4C (g) + 5H2O (g)
Internal combustion engines work by changing chemical energy to kinetic energy, fuelled by the combustion of alkane fuels in oxygen. When this reaction is undergone, so do other unwanted side reactions due to the high pressure and temperature, e.g. the production of nitrogen oxides.
Nitrogen is regularly unreactive but when combined with oxygen, it produces NO and NO2 molecules:
nitrogen + oxygen -> nitrogen (II) oxide
N2 (g) + O2 (g) -> 2NO (g)
and
nitrogen + oxygen -> nitrogen (II) oxide
N2 (g) + 2O2 (g) -> 2NO2 (g)
Sulfur dioxide (SO2) is sometimes present in the exhaust mixture as impurities from crude oil. It is produced when sulfur reacts with oxygen. Nitrogen oxides, carbon dioxide, carbon monoxide, carbon particles, unburnt hydrocarbons, water vapour and sulfur dioxide are all produced in exhaust fumes and are also pollutants that cause problems you need to be aware of for the exam as well as how to get rid of them.
Greenhouse gases contribute to global warming, an important process where infrared radiation from the sun is prevented from escaping back into space by atmospheric gases. On the one hand, some greenhouse gases need to continue this so that the earth can sustain life as it traps heat, however, we do not want the earth’s temperature to increase that much. Global warming is the term given to the increasing average temperature of the earth, which has seen an increase in the last few years due to human activity - burning fossil fuels like alkanes has produced more gases which trap more heat. Examples of greenhouse gases include carbon dioxide, methane and water vapour.

Another pollution problem the earth faces is acid rain. Rain water is already slightly acidic due to the CO2 present in the atmosphere but acid rain is more acidic than this. Nitrogen oxides contribute to acid rain although sulfur dioxide is the main cause. The equation for sulfur dioxide reacting with water in the air to produce oxidised sulfurous acid and therefore sulphuric acid is:
SO2 (g) + H2O (g) + ½ O2 (g) -> H2SO4 (aq)
Acid rain is a problem because it destroys lakes, buildings and vegetation. It is also a global problem because it can fall far from the original source of the pollution.
Photochemical smog is formed from nitrogen oxides, sulfur dioxide and unburnt hydrocarbons that react with sunlight. It mostly forms in industralised cities and causes health problems such as emphysema.
So what can we do about the pollutants?
A good method of stopping pollution is preventing it in the first place, therefore cars have catalytic converters which reduce the amount of carbon monoxide, nitrogen oxides and unburnt hydrocarbons come into the atmosphere by converting them into less toxic gases. Shaped like a honeycomb for increased SA and therefore rate of conversion, platinum and rhodium coat ceramic and act as catalysts for the reactions that take place in an internal combustion engine.
As they pass over the catalyst, they react with each other to form less pollution:
octane + nitrogen (II) oxide -> carbon dioxide + nitrogen + water
C8H18 (g) + 25NO -> 8CO2 (g) + 12 ½ N2 (g) + 9H2O (g)
nitrogen (II) oxide + carbon monoxide -> carbon dioxide + nitrogen
2NO (g) + 2CO (g) -> 2CO2 (g) + N2 (g)
Finally, sulfur dioxide must be dealt with. The first way it is dealt with is by removing it from petrol before it can be burnt, however, this is often not economically favourable for fuels used in power stations. A process called flue gas desulfurisation is used instead.
In this, gases are passed through a wet semi-solid called a slurry that contains calcium oxide or calcium carbonate. These neutralise the acid, due to being bases, to form calcium sulfate which has little commercial value but can be oxidised to produce a more valuable construction material.
calcium oxide + sulfur dioxide -> calcium sulfite
CaO (s) + SO2 (g) -> CaSO3 (s)
calcium carbonate + sulfur dioxide -> calcium sulfite + carbon dioxide
CaCO3 (s) + SO2 (g) -> CaSO3 (s) + CO2 (g)
calcium sulfite + oxygen -> calcium sulfate
CaSO3 (s) + O -> CaSO4 (s)
SUMMARY
Alkanes are used as fuels due to how they can combust easily to release large amounts of heat energy. Combustion is essentially burning something in the presence of oxygen.
Complete combustion occurs when there is a plentiful supply of air. When an alkane is burned in sufficient oxygen, it produces carbon dioxide and water
Remember state symbols in combustion reactions. In addition, reactions can be halved to balance for 1 mole of compounds by using fractions when dealing with the numbers.
Incomplete combustion occurs when there is a limited supply of air. There are two kinds of incomplete combustion.
The first type produces water and carbon monoxide.
Carbon monoxide is dangerous because it is toxic and undetectable due to being smell-free and colourless. It reacts with haemoglobin in your blood to reduce their oxygen-carrying ability and can cause drowsiness, nausea, respiratory failure or death.
The other kind of incomplete combustion occurs in even less oxygen. It produces water and soot (carbon).
Internal combustion engines work by changing chemical energy to kinetic energy, fuelled by the combustion of alkane fuels in oxygen. When this reaction is undergone, so do other unwanted side reactions due to the high pressure and temperature, e.g. the production of nitrogen oxides.
Nitrogen is regularly unreactive but when combined with oxygen, it produces NO and NO2 molecules:
Sulfur dioxide (SO2) is sometimes present in the exhaust mixture as impurities from crude oil. It is produced when sulfur reacts with oxygen.
Nitrogen oxides, carbon dioxide, carbon monoxide, carbon particles, unburnt hydrocarbons, water vapour and sulfur dioxide are all produced in exhaust fumes and are also pollutants that cause problems you need to be aware of for the exam as well as how to get rid of them.
Greenhouse gases contribute to global warming, an important process where infrared radiation from the sun is prevented from escaping back into space by atmospheric gases. Some greenhouse gases need to continue this so that the earth can sustain life as it traps heat, however, we do not want the earth’s temperature to increase that much. Global warming is the term given to the increasing average temperature of the earth, which has seen an increase in the last few years due to human activity - burning fossil fuels like alkanes has produced more gases which trap more heat.
Another pollution problem the earth faces is acid rain. Nitrogen oxides contribute to acid rain although sulfur dioxide is the main cause.
Acid rain is a problem because it destroys lakes, buildings and vegetation. It is also a global problem because it can fall far from the original source of the pollution.
Photochemical smog is formed from nitrogen oxides, sulfur dioxide and unburnt hydrocarbons that react with sunlight. It mostly forms in industralised cities and causes health problems such as emphysema.
A good method of stopping pollution is preventing it in the first place, therefore cars have catalytic converters which reduce the amount of carbon monoxide, nitrogen oxides and unburnt hydrocarbons come into the atmosphere by converting them into less toxic gases. Shaped like a honeycomb for increased SA and therefore rate of conversion, platinum and rhodium coat ceramic and act as catalysts for the reactions that take place in an internal combustion engine.
As they pass over the catalyst, they react with each other to form less pollution.
octane + nitrogen (II) oxide -> carbon dioxide + nitrogen + water
C8H18 (g) + 25NO -> 8CO2 (g) + 12 ½ N2 (g) + 9H2O (g)
nitrogen (II) oxide + carbon monoxide -> carbon dioxide + nitrogen
2NO (g) + 2CO (g) -> 2CO2 (g) + N2 (g)
Finally, sulfur dioxide must be dealt with. The first way it is dealt with is by removing it from petrol before it can be burnt, however, this is often not economically favourable for fuels used in power stations. A process called flue gas desulfurisation is used instead.
In this, gases are passed through a wet semi-solid called a slurry that contains calcium oxide or calcium carbonate. Since they are bases, these neutralise the acid to form calcium sulfate which has little commercial value but can be oxidised to produce a more valuable construction material.
Happy studying!

Follow @productive-tips for more tips and content like this posted daily! Handpicked and curated with love :)
🌻 little habits/things to do more of 🌻
dailies
make your bed. (no, really.)
set your top 3 to-dos for the day.
do your top 3 to-dos for the day. (heh)
stretch.
unpack your bag when you get home.
prepare your things for the next day before sleeping.
skincare. (your basic cleanse and moisturize)
sweep the floor of your bedroom.
talk to your plants. (if you have plants)
update your financial report/expense tracker.
take a good photo.
meditate.
hug at least three people. (seriously.)
weeklies
polish your school shoes.
mop your bedroom floor.
dare i say, laundry. (don’t put it off!)
exfoliate.
take a leisure walk.
review your past week and plan your next week accordingly. (a part of your routine may not be working–try something new)
make a piece of art. (a sketch, a collage, a quote in pretty lettering, a god-awful poem..)
sanitize your gadgets. (whip out the wet tissue and wipe away at your phone, your laptop, your mouse, your earphones–just don’t forget to IMMEDIATELY follow that up with a dry cloth to prevent fogging and short circuits)
watch a TED Talk.
make a new playlist.
monthlies
wash your bag.
wash your shoes.
change the sheets of your bed and your pillows.
clip your nails. (honestly)
wax/shave. (if you want. i just really like how fresh my skin feels after i torture it with razors and wax strips)
wipe your shelves/the tops of your furniture clean. (try to avoid dusting. it just scatters the dirt everywhere. use a damp cloth instead)
see if there’s anything in your storage that you don’t need/want anymore and give stuff away or sell them.
review your month and plan the next one accordingly. (just like your weeks. remember to refer to your Life Goal/Year’s Goals page)
finish reading at least one book. (and review it!)
discover new songs.
- 🍂
Halogenoalkanes
Halogenoalkanes are a homologous series of saturated carbon compounds that contain one or more halogen atoms. They are used as refrigerants, solvents, flame retardants, anaesthetics and pharmaceuticals but their use has been restricted in recent years due to their link to pollution and the destruction of the ozone layer.
They contain the functional group C-X where X represents a halogen atom, F,Cl, Br or I. The general formula of the series is CnH2n+1X.
The C-X bond is polar because the halogen atom is more electronegative than the C atom. The electronegativity decreases as you go down group 7 therefore the bond becomes less polar. Flourine has a 4.0 EN whereas iodine has a 2.5 EN meaning it is almost non-polar.
The two types of intermolecular forces between halogenoalkane molecules are Van Der Waals and permanent dipole-dipole interactions. As the carbon chain length increases, the intermolecular forces (due to VDWs) increase as the relative atomic mass increases due to more electrons creating induced dipoles. Therefore the boiling point of the halogenoalkanes increases since more forces must be broken.
Branched chains have lower boiling points than chains of the same length and halogen because the VDWs are working across a greater distance and are therefore weaker.
When the carbon chain length is kept the same, but the halogen atom is changed, despite the effect of the changing polar bond on the permanent dipole-dipole interactions, the changing VDWs have a greater effect on the boiling point. Therefore as RAM increases, the boiling point increases meaning an iodoalkane has a greater boiling point than a bromoalkane if they have the same carbon chain length.
Halogenoalkanes are insoluble or only slightly soluable in water despite their polar nature. They are soluble in organic solvents such as ethanol and can be used as dry cleaning agents because they can mix with other hydrocarbons.
Summary
Halogenoalkanes are saturated carbon compounds with one or more halogen atoms. Their general formula is CnH2n+1X, where X is a halogen. Their functional group is therefore C-X.
They are used as refrigerants, solvents, pharmaceuticals and anaesthetics but have been restricted due to their link to the depletion of the ozone layer.
C-X bonds are polar due to the halogen being more electronegative than the carbon. The polarity of the bond decreases down group 7.
Van der Waals and permanent dipole-dipole interactions are the intermolecular forces in halogenoalkanes.
When carbon chain length increases, boiling points increase due to RAM increasing and the number of Van Der Waals increasing too.
In branched halogenoalkanes, Van Der Waals are working across a greater distance therefore attraction is weaker and boiling points are lower than an identical unbranched chain.
When the halogen is changed, the boiling point increases down the group due to the effect of a greater RAM - more VDWs mean more intermolecular forces to break.
Halogenoalkanes are insoluble in water but soluble in organic solvents like ethanol.
Bonus: free radical substitution reactions in the ozone layer
Ozone, O3, is an allotrope of oxygen that is usually found in the stratosphere above the surface of the Earth. The ozone layer prevents harmful rays of ultraviolet light from reaching the Earth by enhancing the absorption of UV light by nitrogen and oxygen. UV light causes sunburn, cataracts and skin cancer but is also essential in vitamin D production. Scientists have observed a depletion in the ozone layer protecting us and have linked it to photochemical chain reactions by halogen free radicals, sourced from halogenoalkanes which were used a solvents, propellants and refrigerants at the time.
CFCs cause the greatest destruction due to their chlorine free radicals. CFCs – chloroflouroalkanes – were once valued for their lack of toxicity and their non-flammability. This stability means that they do not degrade and instead diffuse into the stratosphere where UV light breaks down the C-Cl bond and produces chlorine free radicals.
RCF2Cl UV light —> RCF2● + Cl●
Chlorine free radicals then react with ozone, decomposing it to form oxygen.
Cl● + O3 —> ClO● + O2
Chlorine radical is then reformed by reacting with more ozone molecules.
ClO● + O3 —-> 2O2 + Cl●
It is estimated that one chlorine free radical can decompose 100 000 molecules of ozone. The overall equation is:
2O3 —-> 3O2
200 countries pledged to phase of the production of ozone depleting agents in Montreal, leading to a search for alternatives. Chemists have developed and synthesised alternative chlorine-free compounds that do not deplete the ozone layer such as hydroflurocarbons (HFCs) like trifluromethane, CHF3.
SUMMARY
Ozone, found in the stratosphere, protects us from harmful UV light which can cause cataracts, skin cancer and sunburn.
Ozone depletion has been linked to the use of halogenoalkanes due to their halogen free radicals.
CFCs were good chemicals to use because they have low toxicity and were non-flammable. The fact they don’t degrade means they diffuse into the stratosphere.
Chlorine free radicals are made when CFCs are broken down by UV light.
These go on to react with ozone to produce oxygen.
Chlorine free radicals are then reformed by reacting with more ozone.
It is a chain reaction that can deplete over 100 000 molecules of ozone.
There is a 200 country ban on their use and scientists have developed alternatives like hydrofluorocarbons to replace them
Happy studying!
Covalent Bonds: Sharing Is Caring!
Welcome to my second out of three posts on bonding - ionic, covalent and metallic. This post also covers the coordinate/ dative bond which I can’t remember if I’ve covered before. Only one more of this series left! Find the others here.
Covalent bonding involves one or more shared pairs of electrons between two atoms. These can be found in simple molecular elements and compounds like CO2 , macromolecular structures like diamond and molecular ions such as ammonium. Covalent bonds mostly occur between non-metals but sometimes metals can form covalent bonds.
Single covalent bonds share just one pair of electrons. Double covalent bonds share two. Triple covalent bonds share three.
Each atom usually provides one electron – unpaired in the orbital – in the bond. The number of unpaired electrons in an atom usually shows how many bonds it can make but sometimes atoms promote electrons to fit in more. Covalent bonds are represented with lines between the atoms – double and triple bonds represented with two and three lines respectively.
Dot and cross diagrams show the arrangement of electrons in covalent bonds. They use dots and crosses to demonstrate that the electrons come from different places and often only the outer shell is shown.

The simple explanation as to how atoms form covalent bonds is that one unpaired electron in the orbital of one atom overlaps with one in another atom. Sometimes atoms promote electrons in the same energy level to form more covalent bonds. For example, if an atom wants to make three covalent bonds but has a full 3s2 shell and a 3p1 shell, it can promote one of its 3s2 electrons so that an electron from the other atoms can fill the 3s shell and pair with the new 3p2 shell.
Sometimes promotion does not occur and that means different compounds can be made such as PCl3 or PCl5.

A lone pair of electrons is a pair of electrons from the same energy sub-level uninvolved in bonding. Sometimes these can form something called a coordinate bond, which contains a shared pair of electrons where both come from one atom. The lone pair of electrons is “donated” into the empty orbital of another atom to form a coordinate bond.

This is an example of a coordinate (sometimes called dative) bond between ammonia and a H+ ion which has an empty orbital. The lone pair on the ammonia overlaps with this H+ ion and donates its electrons. Both electrons come from the ammonia’s lone pair so it is a coordinate bond. This is demonstrated with an arrow. The diagram is missing an overall charge of + on the ammonium ion it produces. Coordinate bonds act the same as covalent bonds.
Once you have your covalent bonds, you need to know about covalent substances and their properties. There are two types of covalent substance: simple covalent (molecular) and macromolecular (giant covalent).
Molecular simply means that the formula for the compound or element describes exactly how many atoms are in one molecule, e.g. H2O. Molecular covalent crystalline substances usually exist as single molecules such as iodine or oxygen. They are usually gases or liquids at room temperature but can be low melting point solids.
Solid molecular covalent solids are crystalline so can be called molecular covalent crystals. Iodine and ice are examples of these. Iodine (shown below) has a regular arrangement which makes it a crystalline substance and water, as ice, has a crystalline structure as well.

The properties of these crystals are that they have low melting points, are very brittle due to the lack of strong bonds holding them together and also do not conduct electricity since no ions are present.
The other kind of covalent substance you need to know is macromolecular. This includes giant covalent structures such as diamond or graphite, which are allotropes of carbon. Non-metallic elements and compounds usually form these crystalline structures with a regular arrangement of atoms.
Allotropes are different forms of the same element in the same physical state.
Diamond is the hardest naturally occurring substance on earth therefore is good for cutting glass and drilling and mining. It has a high melting point due to the many covalent bonds which require a lot of energy to break. Each carbon has four of these bonds joining it to four others in a tetrahedral arrangement with a bond angle of 109.5 degrees and it does not conduct electricity or heat because there are no ions free to move.
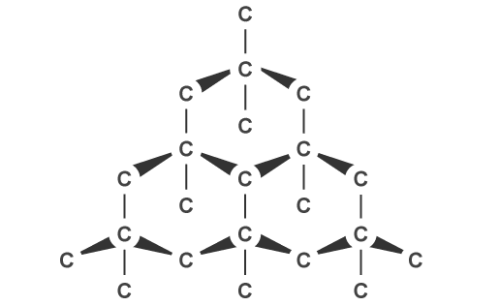
Graphite, on the other hand, can conduct electricity. This is because it has delocalised electrons between the layers which move and carry charge. Carbon atoms within the structure are only bonded to three others in a hexagonal arrangement with a bond angle of 120 degrees. Since only three of carbon’s unpaired electrons are used in bonding, the fourth becomes delocalised and moves between the layers of graphite causing weak attractions, explaining why it can conduct electricity.

Graphite’s layered structure and the weak forces of attractions between it make it a good lubricant and ideal for pencil lead because the layers can slide over each other. The attractions can be broken easily but the covalent bonds within the layers give graphite a high melting point due to the amount of energy needed to break them.
SUMMARY
Covalent bonding involves one or more shared pairs of electrons between two atoms. Covalent bonds mostly occur between non-metals but sometimes metals can form covalent bonds.
Single covalent bonds share just one pair of electrons. Double covalent bonds share two. Triple covalent bonds share three.
Each atom usually provides one electron – unpaired in the orbital – in the bond. The number of unpaired electrons in an atom usually shows how many bonds it can make but sometimes atoms promote electrons to fit in more. Covalent bonds are represented with lines between the atoms.
Dot and cross diagrams use dots and crosses to demonstrate that the electrons come from different places and often only the outer shell is shown.
The simple explanation as to how atoms form covalent bonds is that one unpaired electron in the orbital of one atom overlaps with one in another atom. Sometimes atoms promote electrons in the same energy level to form more covalent bonds.
Sometimes promotion does not occur and that means different compounds can be made such as PCl3 or PCl5.
A lone pair of electrons is a pair of electrons from the same energy sub-level uninvolved in bonding. Sometimes these can form something called a coordinate bond, which contains a shared pair of electrons where both come from one atom. The lone pair of electrons is “donated” into the empty orbital of another atom to form a coordinate bond.
The formation of ammonium is an example of this.
There are two types of covalent substance: simple covalent (molecular) and macromolecular (giant covalent).
Molecular simply means that the formula for the compound or element describes exactly how many atoms are in one molecule, e.g. H2O. Molecular covalent crystalline substances usually exist as single molecules such as iodine or oxygen. They are usually gases or liquids at room temperature but can be low melting point solids.
Solid molecular covalent solids are crystalline so can be called molecular covalent crystals. Iodine and ice are examples of these.
The properties of these crystals are that they have low melting points, are very brittle due to the lack of strong bonds holding them together and also do not conduct electricity since no ions are present.
Giant covalent structures such as diamond or graphite are allotropes of carbon. Allotropes are different forms of the same element in the same physical state.
Diamond has a high melting point due to the many covalent bonds which require a lot of energy to break. Each carbon has four of these bonds joining it to four others in a tetrahedral arrangement with a bond angle of 109.5 degrees and it does not conduct electricity or heat because there are no ions free to move.
Graphite can conduct electricity. This is because it has delocalised electrons between the layers which move and carry charge. Carbon atoms within the structure are only bonded to three others in a hexagonal arrangement with a bond angle of 120 degrees. Since only three of carbon’s unpaired electrons are used in bonding, the fourth becomes delocalised and moves between the layers of graphite causing weak attractions, explaining why it can conduct electricity.
Graphite’s layered structure and the weak forces of attractions between it make it a good lubricant and ideal for pencil lead because the layers can slide over each other. The attractions can be broken easily but the covalent bonds within the layers give graphite a high melting point due to the amount of energy needed to break them.
Happy studying!
Alkanes: Crash Course Organic Chemistry #6:
Alkanes are kind of the wallflowers of organic chemistry, but they still have important functions in the world around us. In this episode of Crash Course Organic Chemistry we’re building our knowledge of organic molecules by learning all about these so called couch potatoes from how they are separated from crude oil to how to use Newman projections to predict torsional strain and steric hinderance. We’ll also learn the names of some common conformers and get an introduction to cycloalkanes.
-
 tajgernewdemon liked this · 8 months ago
tajgernewdemon liked this · 8 months ago -
 20darksideofthemoon02 liked this · 1 year ago
20darksideofthemoon02 liked this · 1 year ago -
 absolut09 liked this · 3 years ago
absolut09 liked this · 3 years ago -
 solely-02 liked this · 3 years ago
solely-02 liked this · 3 years ago -
 grouchomarx86 liked this · 3 years ago
grouchomarx86 liked this · 3 years ago -
 cg10694 liked this · 4 years ago
cg10694 liked this · 4 years ago -
 trinalli liked this · 4 years ago
trinalli liked this · 4 years ago -
 taken-aurally liked this · 4 years ago
taken-aurally liked this · 4 years ago -
 symbiotic-science reblogged this · 4 years ago
symbiotic-science reblogged this · 4 years ago -
 pleasurehunter2000 liked this · 4 years ago
pleasurehunter2000 liked this · 4 years ago -
 birdflu2k11 liked this · 4 years ago
birdflu2k11 liked this · 4 years ago -
 awesomevaliantcollectionfacelove liked this · 4 years ago
awesomevaliantcollectionfacelove liked this · 4 years ago -
 rusconie liked this · 4 years ago
rusconie liked this · 4 years ago -
 prommethium liked this · 4 years ago
prommethium liked this · 4 years ago -
 jaemhan liked this · 4 years ago
jaemhan liked this · 4 years ago -
 concon667 liked this · 4 years ago
concon667 liked this · 4 years ago -
 avivasue liked this · 4 years ago
avivasue liked this · 4 years ago -
 engineeringworldhealth liked this · 4 years ago
engineeringworldhealth liked this · 4 years ago -
 ericalagu liked this · 4 years ago
ericalagu liked this · 4 years ago -
 roughedges151 reblogged this · 4 years ago
roughedges151 reblogged this · 4 years ago -
 amateurchemstudent reblogged this · 4 years ago
amateurchemstudent reblogged this · 4 years ago -
 amateurchemstudent liked this · 4 years ago
amateurchemstudent liked this · 4 years ago -
 xmuinoi liked this · 4 years ago
xmuinoi liked this · 4 years ago -
 vaguely-none liked this · 4 years ago
vaguely-none liked this · 4 years ago -
 endocryptor reblogged this · 4 years ago
endocryptor reblogged this · 4 years ago -
 endocryptor liked this · 4 years ago
endocryptor liked this · 4 years ago -
 theaxiomofhope reblogged this · 4 years ago
theaxiomofhope reblogged this · 4 years ago -
 theaxiomofhope liked this · 4 years ago
theaxiomofhope liked this · 4 years ago -
 garuad liked this · 4 years ago
garuad liked this · 4 years ago -
 leirathemartian reblogged this · 4 years ago
leirathemartian reblogged this · 4 years ago -
 leirathemartian liked this · 4 years ago
leirathemartian liked this · 4 years ago -
 jesmx5 liked this · 4 years ago
jesmx5 liked this · 4 years ago -
 piningmarten liked this · 4 years ago
piningmarten liked this · 4 years ago -
 cumagloia liked this · 4 years ago
cumagloia liked this · 4 years ago -
 whatwwwwwww reblogged this · 4 years ago
whatwwwwwww reblogged this · 4 years ago -
 ffbuddy68 liked this · 4 years ago
ffbuddy68 liked this · 4 years ago -
 raginrayguns reblogged this · 4 years ago
raginrayguns reblogged this · 4 years ago -
 girlactionfigure liked this · 4 years ago
girlactionfigure liked this · 4 years ago

