Periodic Table Showing The Cosmogenic Origin Of Each Element.

Periodic table showing the cosmogenic origin of each element.
Via APOD
Original source: Wikimedia Commons (Nucleosynthesis periodic table)
More Posts from Secretagentpeptidebond and Others
Silica nanoparticles could be used to repair damaged teeth

Researchers at the University of Birmingham have shown how the development of coated silica nanoparticles could be used in restorative treatment of sensitive teeth and preventing the onset of tooth decay.
The study, published in the Journal of Dentistry, shows how sub-micron silica particles can be prepared to deliver important compounds into damaged teeth through tubules in the dentine.
The tiny particles can be bound to compounds ranging from calcium tooth building materials to antimicrobials that prevent infection.
Professor Damien Walmsley, from the School of Dentistry at the University of Birmingham, explained, “The dentine of our teeth have numerous microscopic holes, which are the entrances to tubules that run through to the nerve. When your outer enamel is breached, the exposure of these tubules is really noticeable. If you drink something cold, you can feel the sensitivity in your teeth because these tubules run directly through to the nerve and the soft tissue of the tooth.”
“Our plan was to use target those same tubules with a multifunctional agent that can help repair and restore the tooth, while protecting it against further infection that could penetrate the pulp and cause irreversible damage.”
The aim of restorative agents is to increase the mineral content of both the enamel and dentine, with the particles acting like seeds for further growth that would close the tubules.
Previous attempts have used compounds of calcium fluoride, combinations of carbonate-hydroxypatite nanocrystals and bioactive glass, but all have seen limited success as they are liable to aggregate on delivery to the tubules. This prevents them from being able to enter the opening which is only 1 to 4 microns in width.
However, the Birmingham team turned to sub-micron silica particles that had been prepared with a surface coating to reduce the chance of aggregation.
When observed using high definition SEM (Scanning Electron Microsopy), the researchers saw promising signs that suggested that the aggregation obstacle had been overcome.
Professor Zoe Pikramenou, from the School of Chemistry at the University of Birmingham, said, “These silica particles are available in a range of sizes, from nanometre to sub-micron, without altering their porous nature. It is this that makes them an ideal container for calcium based compounds to restore the teeth, and antibacterial compounds to protect them. All we needed to do was find the right way of coating them to get them to their target. We have found that different coatings does change the way that they interact with the tooth surface.”
“We tested a number of different options to see which would allow for the highest level particle penetration into the tubules, and identified a hydrophobic surface coating that provides real hope for the development of an effective agent.”
Our next steps are to optimise the coatings and then see how effective the particles are blocking the communication with the inside of the tooth. The ultimate aim is to provide relief from the pain of sensitivity.
University of Birmingham
Nanotechnology World Association


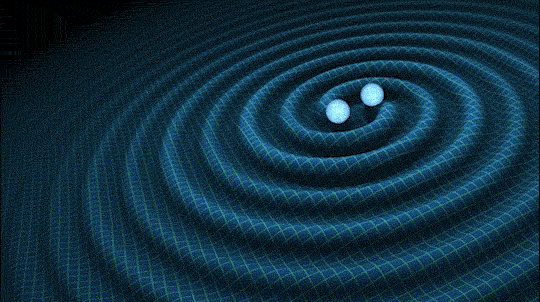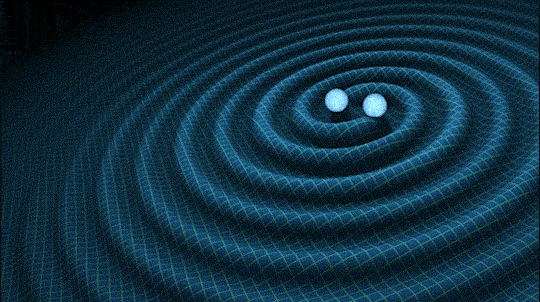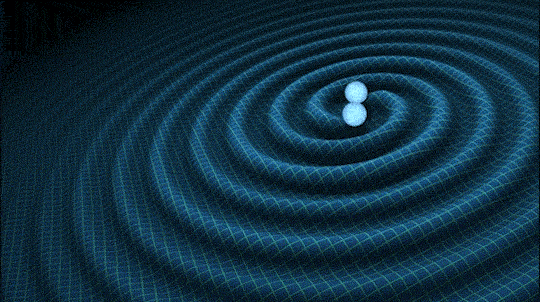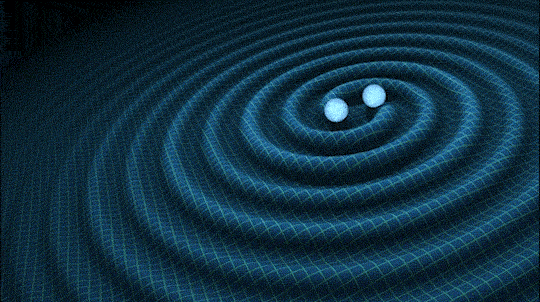



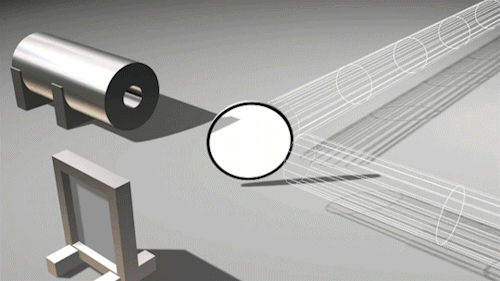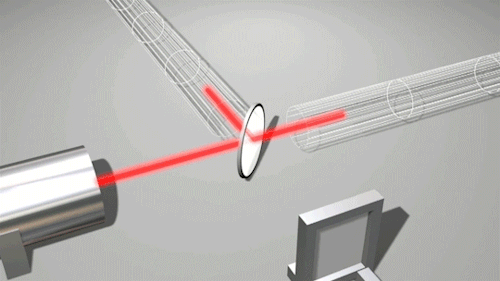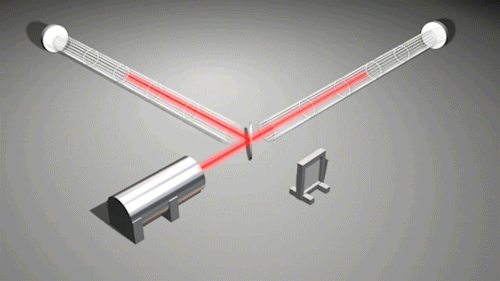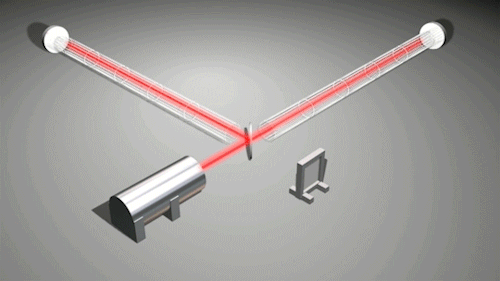


This is very cool and a pretty big deal. Find out why.
Stupid Lab Mistakes: a Constant Across All Levels of Experience
So today was day one of a tyrosinase lab. We had to centrifuge our organic matter sample (homogenized mushroom gills) to separate the soluble proteins from the rest of the tissue, and we counterbalanced our sample with an identical centrifuge tube of equal weight filled with water. We did all of this under the supervision of a TA and our professor.
My lab partners and I have used centrifuges many times before, but what we (and the instructors) failed to notice was that these particular tubes required an adapter for this particular centrifuge. We were spinning it up to about 7000 rcf when we heard a muffled “bang,” then the centrifuge slowed to a stop. When we opened it up, we discovered that the water tube had EXPLODED, shattering into a hundred little plastic pieces. the tube containing our organic sample slurry was thankfully intact, but it was badly warped and cracked. We spent the next 20 minutes or so carefully wiping water off every nook and cranny of the centrifuge interior, thanking our lucky stars that it wasn’t mushroom gloop.
I keep thinking that gaining more practical lab experience will save me from this kind of thing, but if the three different generations of chemists present couldn’t keep it from happening then there is no hope. These incidents are the things that add unexpected excitement to my life, though, so I suppose it’s not all bad.
The use of 3-D printers has opened up the possibility of on-demand implants, prosthetics, and medical devices. This week, scientists reported that they were able to 3-D-print the first stable ear, bone, and muscle structures out of living cells and implant them in mice. The results were published in Nature Biotechnology. Anthony Atala, the director of the Wake Forest Institute for Regenerative Medicine and an author on that paper, describes the challenges of 3-D printing living cells and how the technology could be used in bioengineering body parts.

Copper essential for burning fat, researchers find
Though small amounts of copper are essential to health - oysters, liver, beans and nuts are good sources - copper’s role in metabolism has been unclear: Some studies found that it boosted fat burning, others that it depressed it.
University of California, Berkeley, Lawrence Berkeley National Laboratory and Howard Hughes Medical Institute researchers have now clarified the critical role that copper plays in nutrition: It helps move fat out of fat cells - called adipocytes - and into the blood stream for use as energy.
Without enough copper, fat builds up in fat cells without being utilized, said Christopher Chang, the Class of 1942 Chair and a professor of chemistry and of molecular and cell biology at UC Berkeley.
“Unlike other studies that link copper levels both to increased or decreased fat metabolism, our study shows definitively how it works - it’s a signal that turns on fat cells,” said Chang, who also is a faculty scientist at Berkeley Lab and a Howard Hughes Medical Institute investigator. “If we could find a way to burn fat more efficiently, this could be a big contribution to dealing with obesity and diabetes.”
The new study appeared online this week, and will be published in the July print issue of the journal Nature Chemical Biology.
“Copper regulates cyclic-AMP-dependent lipolysis” by Lakshmi Krishnamoorthy, Joseph A Cotruvo Jr, Jefferson Chan, Harini Kaluarachchi, Abigael Muchenditsi, Venkata S Pendyala, Shang Jia, Allegra T Aron, Cheri M Ackerman, Mark N Vander Wal, Timothy Guan, Lukas P Smaga, Samouil L Farhi, Elizabeth J New, Svetlana Lutsenko and Christopher J Chang in Nature Chemical Biology. Published online June 6 2016 doi:10.1038/nchembio.2098
Caption: The crystal structure of the cAMP-degrading enzyme phosphodiesterase PDE3B, showing two magnesium atoms (green) in the active site. Copper binds one of the amino acid residues in the pink loop at the left, blocking the activity of the enzyme. Credit: Lakshmipriya Krishnamoorthy and Joseph Cotruvo Jr., UC Berkeley


In this video lichtenburg figures are burned into wood using a microwave oven transformer. The results are spectacular. (Video) Facebook | Instagram | Scary Story Site
Jane Goodall teaches John Oliver how to fight, eat bananas like a chimpanzee
-
 garfinski liked this · 1 year ago
garfinski liked this · 1 year ago -
 deusexxxmachina reblogged this · 3 years ago
deusexxxmachina reblogged this · 3 years ago -
 lem8n liked this · 3 years ago
lem8n liked this · 3 years ago -
 shinypersonbageleclipse liked this · 3 years ago
shinypersonbageleclipse liked this · 3 years ago -
 heroofashesnot liked this · 4 years ago
heroofashesnot liked this · 4 years ago -
 iingyt liked this · 6 years ago
iingyt liked this · 6 years ago -
 please-bring-me-pizza liked this · 6 years ago
please-bring-me-pizza liked this · 6 years ago -
 pchpck-blog liked this · 6 years ago
pchpck-blog liked this · 6 years ago -
 neuvirtualoasis reblogged this · 6 years ago
neuvirtualoasis reblogged this · 6 years ago -
 bearrabbitt reblogged this · 6 years ago
bearrabbitt reblogged this · 6 years ago -
 selfish-giant reblogged this · 6 years ago
selfish-giant reblogged this · 6 years ago -
 recklessmushroom liked this · 6 years ago
recklessmushroom liked this · 6 years ago -
 friendly-asteroid liked this · 6 years ago
friendly-asteroid liked this · 6 years ago -
 thewinterwombat reblogged this · 6 years ago
thewinterwombat reblogged this · 6 years ago -
 thewinterwombat liked this · 6 years ago
thewinterwombat liked this · 6 years ago -
 zaurac reblogged this · 6 years ago
zaurac reblogged this · 6 years ago -
 inexpressib1e liked this · 7 years ago
inexpressib1e liked this · 7 years ago -
 foreverthemusiclives liked this · 7 years ago
foreverthemusiclives liked this · 7 years ago -
 robunix2009 reblogged this · 7 years ago
robunix2009 reblogged this · 7 years ago -
 littolteapot reblogged this · 7 years ago
littolteapot reblogged this · 7 years ago -
 littolteapot liked this · 7 years ago
littolteapot liked this · 7 years ago -
 luoya0810 liked this · 7 years ago
luoya0810 liked this · 7 years ago -
 tango-smarts reblogged this · 7 years ago
tango-smarts reblogged this · 7 years ago -
 bedroom-tango liked this · 7 years ago
bedroom-tango liked this · 7 years ago -
 herinternetdaze liked this · 7 years ago
herinternetdaze liked this · 7 years ago -
 in-stars-we-trust liked this · 7 years ago
in-stars-we-trust liked this · 7 years ago -
 vvitchyybitch liked this · 7 years ago
vvitchyybitch liked this · 7 years ago -
 tothesunandalltheshitinbetween liked this · 7 years ago
tothesunandalltheshitinbetween liked this · 7 years ago -
 happiestm reblogged this · 7 years ago
happiestm reblogged this · 7 years ago -
 affectionatelydead reblogged this · 7 years ago
affectionatelydead reblogged this · 7 years ago -
 affectionatelydead liked this · 7 years ago
affectionatelydead liked this · 7 years ago -
 laurelei-witchery reblogged this · 7 years ago
laurelei-witchery reblogged this · 7 years ago -
 helleborehexgrove liked this · 7 years ago
helleborehexgrove liked this · 7 years ago -
 lavender-tarot reblogged this · 7 years ago
lavender-tarot reblogged this · 7 years ago



