Curate, connect, and discover
Study Help - Blog Posts
Enthalpy - a thermodynamic property
When I first learned about enthalpy, I was shocked - it felt more like a physics lesson than a chemistry lesson. The thought of learning more about thermodynamics than my basic understanding from my many science lessons in lower school made me bored out of my mind. But enthalpy is actually pretty interesting, once you get your head around it…
Reactions which release heat to their surroundings are described to be exothermic. These are reactions like combustion reactions, oxidation reactions and neutralisation reactions. Endothermic reactions take in heat from their surroundings, such as in thermal decomposition. Reversible reactions are endothermic in one direction and exothermic in the other.
These facts are important when you start to look at enthalpy. Enthalpy is basically a thermodynamic property linked to internal energy, represented by a capital H. This is pretty much the energy released in bond breaking and made in bond making. We usually measure a change in enthalpy, represented by ∆H. ∆H = enthalpy of the products (H1) - enthalpy of the reactants (H2). This is because we cannot measure enthalpy directly.
In exothermic reactions, ∆H is negative whereas in endothermic reactions, ∆H is positive.
∆H is always measured under standard conditions of 298K and 100kPa.
In reversible reactions, the ∆H value is the same numerical value forwards and backwards but the sign is reversed. For example, in a forward exothermic reaction, the ∆H value would be -ve but in the backwards reaction (endothermic) the ∆H would be +ve.
Reaction profiles are diagrams of enthalpy levels of reactants and products in a chemical reaction. X axis is enthalpy rather than ∆H and the Y axis is the progress of reaction, reaction coordinate or extent of reaction. Two horizontal lines show the enthalpy of reactants and products with the reactants on the left and the products on the right. These should be labelled with their names or formulae.
In an endothermic reaction, product lines are higher enthalpy values than reactants. In an exothermic reaction, product lines are lower enthalpy values than reactants. The difference between product and reactant lines is labelled as ∆H. Values are measured in kJ mol-1.
Reaction pathways are shown with lines from the reactants to the products on enthalpy level diagrams. This shows the “journey” that the enthalpy takes during a reaction. They require an input of energy to break bonds before new bonds can form the products. The activation energy is the peak of the pathway above the enthalpy of reactants. It is the minimum amount of energy that reactants must have to react.

Standard enthalpy values are the ∆H values for enthalpy changes of specific reactions measured under standard conditions, represented by ⊖. There are three of these:
1. Standard enthalpy of reaction ( ΔHr⊖ )
The enthalpy change when substances react under standard conditions in quantities given by the equation for the reaction.
2. Standard enthalpy of formation ( ΔfH⊖ )
The enthalpy change when 1 mole of a compound is formed from its constitutent elements with all reactants and products in standard states under standard conditions.
The enthalpy of formation for an element is zero is it is in it’s standard state for example, O2 enthalpy is zero.
3. Standard enthalpy of combustion ( ΔcH⊖ )
The enthalpy change when 1 mole of a substance is burned completely in excess oxygen with all reactants and products in their standard states under standard conditions.
Values for standard enthalpy of formation and combustion must be kept to per mole of what they refer.
Summary
Reactions which release heat to their surroundings are described to be exothermic. Endothermic reactions take in heat from their surroundings, such as in thermal decomposition.
Reversible reactions are endothermic in one direction and exothermic in the other.
Enthalpy is a thermodynamic property linked to internal energy, represented by a capital H. We usually measure a change in enthalpy, represented by ∆H.
∆H = enthalpy of the products (H1) - enthalpy of the reactants (H2). We cannot measure enthalpy directly.
In exothermic reactions, ∆H is negative whereas in endothermic reactions, ∆H is positive.
∆H is always measured under standard conditions of 298K and 100kPa.
In reversible reactions, the ∆H value is the same numerical value forwards and backwards but the sign is reversed.
Reaction profiles are diagrams of enthalpy levels of reactants and products in a chemical reaction. They
In an endothermic reaction, product lines are higher enthalpy values than reactants. In an exothermic reaction, product lines are lower enthalpy values than reactants.
The difference between product and reactant lines is labelled as ∆H.
Values are measured in kJ mol-1.
Reaction pathways are shown with lines from the reactants to the products on enthalpy level diagrams. They plot enthalpy against reaction progress.
Reactions require an input of energy to break bonds before new bonds can form the products. The activation energy is the peak of the pathway above the enthalpy of reactants. It is the minimum amount of energy that reactants must have to react.
Standard enthalpy values are the ∆H values for enthalpy changes of specific reactions measured under standard conditions, represented by ⊖.
Standard enthalpy of reaction ( ΔHr⊖ ) is the enthalpy change when substances react under standard conditions in quantities given by the equation for the reaction.
Standard enthalpy of formation ( ΔfH⊖ ) is the enthalpy change when 1 mole of a compound is formed from its constitutent elements with all reactants and products in standard states under standard conditions.
The enthalpy of formation for an element is zero is it is in it’s standard state.
Standard enthalpy of combustion ( ΔcH⊖ ) is the enthalpy change when 1 mole of a substance is burned completely in excess oxygen with all reactants and products in their standard states under standard conditions.
Values for standard enthalpy of formation and combustion must be kept to per mole of what they refer.
Happy studying!

Just completed and submitted the final version of my Extended Essay !!! °˖✧◝(⁰▿⁰)◜✧˖°
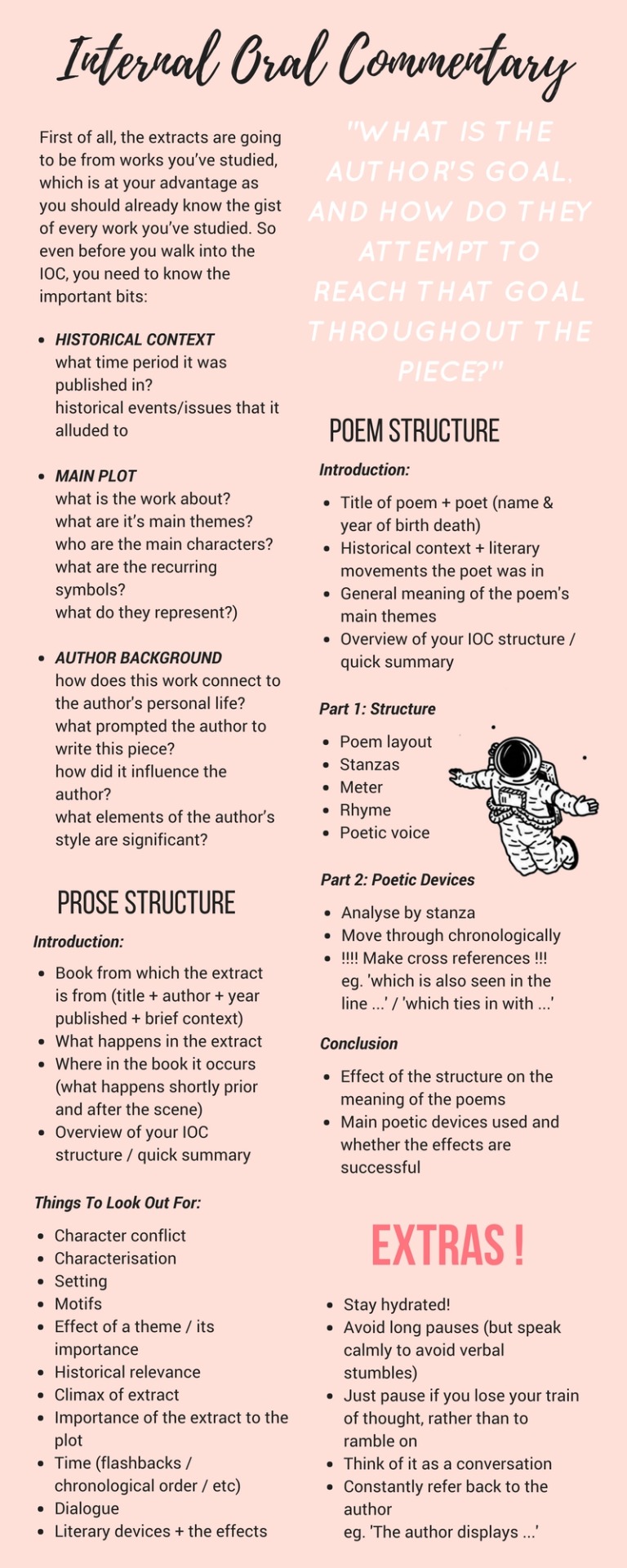
So I compiled lists of words that I found super super useful in making it easier for me to finish any essay !! Here is a masterpost of some sort with transition words + key vocabs grouped together for easy access as well as an IB IOC ‘cheat sheet’ I used for my english LAL orals last year (though most commentaries follow a similar structure so its generalisable) ~
Good luck with your essays !!!
… oops just realised I forgot to bullet point one of the lists
Requests for mood boards or playlists are open again! I need some motivation to get back into studying so please send some in ☺️✨

Day 72/100 days of productivity ☕️🌿
Today wasn’t great but I still got a lot done! I finally did some writing that I’ve been putting off but the deadline is in a few days and I’m not sure if I’m going to finish in time... it’s really annoying because I really didn’t mean to procrastinate this long :/
21st July - What is the best vacation you have ever been on? (note: does not have to have been during summer)
Ah, October a couple of years ago we went to America and it was amazing, we went to Disneyland and saw my aunts and uncles that live there. There were so many amazing coffee shops and I tried a cake shake which was literally a milkshake blended with a cupcake 🧁 I really enjoyed myself then 💫✨

Day 71/100 days of productivity ☕️🌙
Today went well! I met up with my friend at the park and I had a really great time. I also tidied my room, worked out and overall had a really productive day. So far I’ve stuck with my new bullet journal and I’m super proud of it, even if it is quite a simple design. ☺️💫
17th July - What is the most important task that you need to complete this summer?
I think it’ll be preparing for sixth form! Normally we’d have had an introductory week at the end of term but due to corona we’ve been left to ourselves aha, so this summer I’ll be getting everything ready and double checking I have everything I need! ☺️💫

Day 70/100 days of productivity ☕️🌙
Finally committed to a paper bullet journal! It looks pretty good so far and I’m super proud! It’s really therapeutic to fill out. I also got most of my jobs done today! There were a few I missed but I’ll get them finished tomorrow ☺️✨☕️
14th July - Have your plans changed at all because of the pandemic?
Yep! I was supposed to do GCSEs this year but those have been forgotten :/ it’s nice to not do exams but it feels kind of anticlimactic and a bit like the work I did for the last two years DIDNT end up being used properly. However! It did mean I got to relax on my birthday instead of revising, every cloud ⛅️ ☺️✨☕️

Day 69/100 days of productivity 🌿🦋
Today went well! I got a ton of stuff done and was really pleased with myself ☺️ I kind of overslept this morning but not by much so I wasnt too thrown off ☺️✨🌿
13th July - What are your plans for this summer?
There’s still a chance we could go on holiday! We booked ours for the very end of August so it’s still possible as long as we take correct procedures. I’ll also try to meet up with friends and get some back to school shopping done ☺️✨

67/100 days of productivity 💫🥥
Today was good! I tidied my room, worked out and went mini golfing! I also got through loads of my book (it’s so long but the writing is amazing!) 🍋🌿 I’m thinking of starting my bullet journal again, what do you think? 🍯

66/100 days of productivity 🥥🍋
Today was good! I feel super tired but I got through my to do list and sent a few emails. I’ve been toning down how much I do every day more recently since it’s now the summer and I want to relax, even if we didn’t have the exams we still worked super hard these past two years and it’s nice to not have to deal with any more of that! Have you guys met up with any of your friends in person yet?


66/100 days of productivity 💦⚡️
Today went well! I got through everything on my list and had a lovely morning. I’m making more progress with my book! It’s so long but the writing is amazing so it’s worth it ☺️. How are you guys doing today?


Day 65/100 days of productivity 🌙🌸
Ah, picture on the left is one I took in Mallorca. It was so pretty I saw it and felt I had to post it ☺️. Today I did okay! I’ve been eating healthy and I got through way more of my book. I also flicked through old notes and pre alevel notes in order to refresh myself ☺️✨🍃


Day 64/100 days of productivity ☺️🌿✨
Today was good! I got my workout done early and have started prepping for the new school term early so it’s not all done in a rush. I’ve been budgeting for the next few weeks and have made really great progress on my book. How was today for you guys? 🍋🍃✨

Day 64/100 days of productivity 🌸🍃
Had a better day today but I’m still pretty tired. Did some organising in my room and filled in dates in my diary ready for next year. How have you guys been? ✨🌿

Day 63/100 days of productivity ✨☕️
I read some more of my book today! I absolutely adore Stephen King’s writing. Today kinda sucked. My friend’s upset with me and I’ve been feeling super drained. Idk, I let myself eat kinda unhealthily to make myself a little more comfortable but now I feel guilty aha. Anyways! I hope everyone had a lovely day and make sure you stay hydrated!! ✨☔️☕️


Day 62/100 days of productivity 🍇✨
I got a new plant! It’s this adorable cactus and the plant pot is a little moped and it’s the sweetest thing 😍 I also rewrote my writing notes and did pretty well with everything today! 🍃✨


Day 61/100 days of productivity 🌸🍃
Sorry it’s late! I got everything finished and managed to write the first draft of the booklet for my peer teaching programme ☺️


Day 60/100 days of productivity 🌸🍃✨
Today was good! I didn’t eat as healthy as I’d like to have today but no worries, I got some writing done and designed some characters which is super cool! I also got everything on my to do list sorted ☺️✨🌿


Day 59/100 days of productivity 🥥✨🌾
Today went well! I managed to get some reading done as I’d been neglecting my book lately 🥰 I also started up on learning hiragana again! During GCSEs I forgot about it but with all the free time I had I figured it would be good to begin again ☺️✨🌿


Day 58/100 days of productivity 🌸🌿
Happy Father’s Day for my British followers! I had a great day with my dad and I squeezed in some maths and chemistry work 🥰


Day 57/100 days of productivity
Sorry it’s late! This is for yesterday but I was so tired it completely slipped my mind aha 😅 anyways, I had a good day yesterday and got a good amount done ✨🌿🍯

Day 54/100 days of productivity ✨🍃
Did some chemistry and EPQ work today! I wasn’t feeling the best but I made myself keep going since I didn’t want to have all the work pile up. How are you guys doing?


Day 53/100 days of productivity ✨☕️
Not a bad day :)

Day 45/100 days of productivity
Wasn’t doing too great today, I allowed myself to take some time this evening to make peppermint hot chocolate and to just to calm down.
Achievements:
Went for a walk in the park
Planned out my timetable for the next few days
Read some of my books
Finished reading through my essay and completed my list of sources
Not much done today but I really needed to recuperate and get focused for the rest of the week.

Day 44/100 days of productivity
Achievements:
Finished writing the first draft of my language project
Annotated some of my research sources
Set up a new morning routine
Read 4 chapters of my book
Started reading “the miracle morning”
Had an alright day today, feeling a lot better recently ☺️

Day 34/100 days of productivity
Today’s Achievements:
Went for a run
Started reading this weeks book
Set out some books to send to my friend
Put up some photos in my room
Did some painting
Dried some strawberries for tomorrow’s lunch

Day 28/100 days of productivity
Achievements:
Learnt the first two sections of my Spanish speaking presentation
Rehearsed my lines and used different rehearsal techniques to enhance my character
Wrote an email to an office head asking about work experience
Practised the dance from my drama group
Signed up for the revision courses during Easter
Went to an English Language revision session this evening
Had an alright day today, this week feels like it’s dragging on forever though :(
To any GCSE/A level students who have to teach themselves because of the coronavirus outbreak
Everyone is very much focused right now on Years 11 and 13, whose exams have been cancelled, but Years 10 and 12 will more than likely have exams next year that aren’t - and this time off means you’re losing a lot of teaching time. Whatever measures end up being taken for you next year, it’s important that you are still preparing yourselves for the eventuality that every exam goes ahead as normal. Even if it doesn’t, it’s better to be over prepared than underprepared. I imagine you’ll have been given resources to help you with this, but it’s not the same as being at school/college/sixth form.
So, if you need help with chemistry, French, German, biology or maths, I am happy to answer your questions. I took these subjects at A level and achieved A*AAAA, and I achieved 11 A* grades at GCSE. I am now doing a chemistry degree at a Russell Group university. I taught myself the A level maths course in a year, on my own - I know how difficult self-teaching is and I know that there will be people who will struggle more than others with this. I’m not saying this to brag at all; I’m saying that I am more than qualified to give you some tips if you would like them!
I have no doubt that your teachers will be doing their best to help you as well; I am just offering to do what I can because these are unprecedented circumstances. Nobody knows what next year will look like, or even if we’ll be able to return to our respective institutions in time for the new academic year. The best thing you can do for yourselves is to keep your heads down and do the best you can to keep learning, ready for your eventual return.
I know you probably feel a bit lost (or maybe you don’t - lucky you!) without a teacher in front of you to guide you, but if I were in your boat - and to some extent I am, because I still have to think about standing myself in good stead for next year - I’d be looking to do all I could to minimise my disadvantage.
(Obviously I am busy with my own work as well so I won’t be able to respond immediately, but wherever I am available I will try my best to give you a different perspective on a topic, or perhaps point you towards some new resources if I know of any!)
Good luck guys.