It’s Day 5 Of #ChemAdvent – Here’s Why Peppermint Candy Canes Make Your Mouth Feel Cold! Bit.ly/chemadvent2020
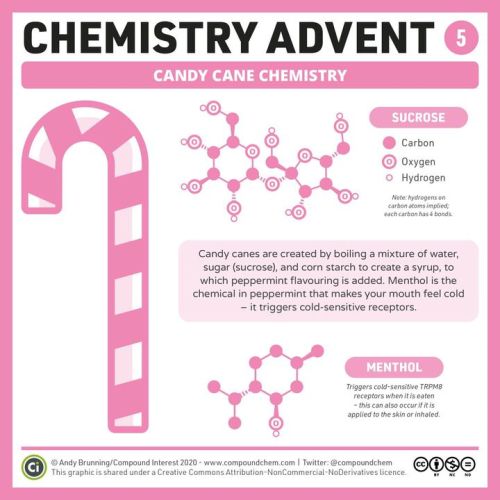
It’s day 5 of #ChemAdvent – here’s why peppermint candy canes make your mouth feel cold! bit.ly/chemadvent2020 https://ift.tt/2JM6bZ7
More Posts from Amateurchemstudent and Others

Linus Pauling was born #OTD in 1901. He’s best known for his work on chemical bonding and the electronegativity scale that bears his name, for which he won a Nobel Prize. Just don’t mention the vitamin C years 😉 https://ift.tt/3uFpNkF https://ift.tt/3r4nm98

finally, some content! this was a quick info graphic I drew up on Procreate to revise for my ochem test tomorrow. disclaimer: I used information from this source (https://www.masterorganicchemistry.com/2010/05/24/imines-and-enamines/) since my own notes are based off lectures I received at my university that I’m not really allowed to share without heavy modification.
general post disclaimer: I’m an undergraduate student studying biochemistry and genetics. Posts are made for the purposes of education, revision and aesthetics. Not all the content I produce can be taken as entirely accurate and I do not take responsibility for errors made as a result of using this resource. Always consult course textbooks and lectures to aid in your specific learning outcomes. Do not repost without the original caption citing any extra references I used to make this post or remove my watermark. Other posts can be found on my blog as-studypeach@tumblr.com. Any problems, feel free to get in touch via my messages.

Alkanes: Saturated Hydrocarbons
So you want to be an organic chemist? Well, learning about hydrocarbons such as alkanes is a good place to start…
Alkanes are a homologous series of hydrocarbons, meaning that each of the series differs by -CH2 and that the compounds contain carbon and hydrogen atoms only. Carbon atoms in alkanes have four bonds which is the maximum a carbon atom can have - this is why the molecule is described to be saturated. Saturated hydrocarbons have only single bonds between the carbon atoms.
The general formula of an alkane is CnH2n+2 where n is the number of carbons. For example, if n = 3, the hydrocarbon formula would be C3H8 or propane. Naming alkanes comes from the number of carbons in the chain structure.
Here are the first three alkanes. Each one differs by -CH2.

Shorter chain alkanes are gases at room temperature, medium ones are liquids and the longer chain alkanes are waxy solids.
Alkanes have these physical properties:
1. They are non-polar due to the tiny difference in electronegativity between the carbon and hydrogen atoms.
2. Only Van der Waals intermolecular forces exist between alkane molecules. The strength of these increase as relative molecular mass increases therefore so does the melting/boiling point.
3. Branched chain alkanes have lower melting and boiling points than straight chain isomers with the same number of carbons. Since atoms are further apart due to a smaller surface area in contact with each other, the strength of the VDWs is decreased.
4. Alkanes are insoluble in water but can dissolve in non-polar liquids like hexane and cyclopentane. Mixtures are separated by fractional distillation or a separating funnel.
The fractional distillation of crude oil, cracking and the combustion equations of the alkanes will be in the next post.
SUMMARY
Alkanes are a homologous series of hydrocarbons. Carbon atoms in alkanes have four bonds which is the maximum a carbon atom can have - this is why the molecule is described to be saturated. Saturated hydrocarbons have only single bonds between the carbon atoms.
The general formula of an alkane is CnH2n+2 where n is the number of carbons.
Shorter chain alkanes are gases at room temperature, medium ones are liquids and the longer chain alkanes are waxy solids.
They are non-polar.
Only Van der Waals intermolecular forces exist between alkane molecules. The strength of these increase as relative molecular mass increases therefore so does the melting/boiling point.
Branched chain alkanes have lower melting and boiling points than straight chain isomers with the same number of carbons.
Alkanes are insoluble in water but can dissolve in non-polar liquids like hexane. Mixtures are separated by fractional distillation or a separating funnel.
Nomenclature - what in the organic chemistry is it?
Organic chemistry is so widely studied it requires a standard system for naming compounds, developed by IUPAC. Nomenclature is simply naming these organic compounds.
So, you want to be an organic chemist? Well, it starts here. Are you ready?
(psst… once you’ve learnt this theory, try a quiz here!)
1. Count your longest continuous chain of carbons.
Bear in mind that some chains may be bent. You’re looking for the longest chain of subsequent carbon atoms. This number correlates to root names that indicate the carbon chain length, listed below:

The second part of naming your base comes from the bonding in the chain. Is it purely single bonds or are there double bonds in there? If you are familiar with carbon chemistry, you’ll know that saturated hydrocarbons are called alkanes and unsaturated hydrocarbons are called alkenes. Therefore, the syllable -ane is used when it has only single bonds and the syllable -ene is used when it has some double bonds. For example:
Sometimes carbon chains exist in rings rather than chains. These have the prefix of -cyclo.
2. Identify your side chains attached to this main carbon and name them.
Side chains are added as prefixes to the root names. Sometimes called substituents, these are basically anything that comes off the carbon chain. Examples of the prefixes are listed below:

There are other prefixes such as fluoro (-F) and chloro (-Cl) which can describe what is coming off the chain.
3. Identify where each side chain is attached and indicate the position by adding a number to the name.
We aim to have numbers as small as possible. For example, if bromine is on the second carbon of a 5-carbon saturated chain, we number it as 2-bromopentane instead of 4-bromopentane, since it would essentially be 2-bromopentane if it was flipped. Locant is the term used for the number which describes the position of the substitute group, e.g. the ‘2′ in 2-chlorobutane is the locant.
Sometimes there are two or more side chains e.g. a methyl group and a chlorine attached to a pentane. In these cases, these rules apply:
1. Names are written alphabetically.
2. A separate number is needed for each side chain or group.
3. Hyphens are used to separate numbers and letters.

This would be named 2-chloro-3-methyl-pentane. This is because the longest chain of carbons is 5 (pentane), the chlorine is on the second carbon (2-chloro) and the methyl group is on the third carbon (3-methyl). It is 2-chloro rather than 4-chloro as we aim to have as small as numbers as possible.
Another variation of this step to be aware of is how many of the same side chains or groups there are, for example, having two methyl groups would be dimethyl rather than solely methyl. Each group must also be given numbers separated by commas to show where each one is located.
The list of these prefixes is found here:

Convention does not usually require mono- to go before a single group or side chain.
4. Number the positions of double bonds if applicable.
Alkenes and other compounds have double bonds. These must be indicated with numbers. For example, pent-2-ene shows that the double bond is between carbon 2 and carbon 3. The number goes in the middle of the original root name e.g. butene, pentene.
(!) Below is a list of functional groups that you may need to study for the AS and A Level chemistry exams. “R” represents misc. carbons. It is important to know that some groups are more prioritised than naming. From the most to least priority: carboxylic acid, ester, acyl chloride, nitrile, aldehyde, ketone, alcohol, amine, alkene, halogenalkane. It is worthwhile learning these.

bigger version here (I suggest downloading and printing it)
But wait, there’s more:
Here are some things to bear in mind when naming organic compounds:
1. The letter ‘e’ is removed when there are two vowels together e.g. propanone rather than propaneone. The ‘e’ isn’t removed when it is next to consonant, e.g. propanenitrile isn’t propannitrile.
2. When compounds contain two different, one is named as part of the unbranched chain and the other is named as a substituent. Which way round this goes depends on the priority.
SUMMARY
Count your longest continuous chain of carbons.
Chains may be bent. You’re looking for the longest chain of subsequent carbon atoms. This number correlates to root names that indicate the carbon chain length, e.g. pentane.
The second part of naming your base comes from the bonding in the chain. Is it purely single bonds or are there double bonds in there? The syllable -ane is used when it has only single bonds and the syllable -ene is used when it has some double bonds.
Rings have the prefix of -cyclo.
Identify your side chains attached to this main carbon and name them.
Side chains are added as prefixes to the root names. Sometimes called substituents, these are basically anything that comes off the carbon chain.
There are other prefixes such as fluoro (-F) and chloro (-Cl) which can describe what is coming off the chain.
Identify where each side chain is attached and indicate the position by adding a number to the name.
We aim to have numbers as small as possible. Locant is the term used for the number which describes the position of the substitute group, e.g. the ‘2′ in 2-chlorobutane is the locant.
Sometimes there are two or more side chains e.g. a methyl group and a chlorine attached to a pentane. In these cases, names are written alphabetically, a separate number is needed for each side chain or group and hyphens are used to separate numbers and letters.
When there are two or more of the same side chains or substituent groups, these must also be given numbers separated by commas to show where each one is located.
Number the positions of double bonds if applicable.
Alkenes and other compounds have double bonds. These must be indicated with numbers. The number goes in the middle of the original root name e.g. butene, pentene.
It is worthwhile learning the other functional groups that can be added on.They have varying priorities.
The letter ‘e’ is removed when there are two vowels together e.g. propanone rather than propaneone. The ‘e’ isn’t removed when it is next to consonant, e.g. propanenitrile isn’t propannitrile.
When compounds contain two different, one is named as part of the unbranched chain and the other is named as a substituent. Which way round this goes depends on the priority.
Happy studying guys!
🌻 little habits/things to do more of 🌻
dailies
make your bed. (no, really.)
set your top 3 to-dos for the day.
do your top 3 to-dos for the day. (heh)
stretch.
unpack your bag when you get home.
prepare your things for the next day before sleeping.
skincare. (your basic cleanse and moisturize)
sweep the floor of your bedroom.
talk to your plants. (if you have plants)
update your financial report/expense tracker.
take a good photo.
meditate.
hug at least three people. (seriously.)
weeklies
polish your school shoes.
mop your bedroom floor.
dare i say, laundry. (don’t put it off!)
exfoliate.
take a leisure walk.
review your past week and plan your next week accordingly. (a part of your routine may not be working–try something new)
make a piece of art. (a sketch, a collage, a quote in pretty lettering, a god-awful poem..)
sanitize your gadgets. (whip out the wet tissue and wipe away at your phone, your laptop, your mouse, your earphones–just don’t forget to IMMEDIATELY follow that up with a dry cloth to prevent fogging and short circuits)
watch a TED Talk.
make a new playlist.
monthlies
wash your bag.
wash your shoes.
change the sheets of your bed and your pillows.
clip your nails. (honestly)
wax/shave. (if you want. i just really like how fresh my skin feels after i torture it with razors and wax strips)
wipe your shelves/the tops of your furniture clean. (try to avoid dusting. it just scatters the dirt everywhere. use a damp cloth instead)
see if there’s anything in your storage that you don’t need/want anymore and give stuff away or sell them.
review your month and plan the next one accordingly. (just like your weeks. remember to refer to your Life Goal/Year’s Goals page)
finish reading at least one book. (and review it!)
discover new songs.
- 🍂
Polarity, Resonance, and Electron Pushing: Crash Course Organic Chemistry #10:
We’ve all heard the phrase “opposites attract.” It may or may not be true for people, but it’s definitely true in organic chemistry. In this episode of Crash Course Organic Chemistry, we’re learning about electronegativity, polarity, resonance structures, and resonance hybrids. We’ll practice a very important skill for this course that will help us avoid a lot of memorization in the future: electron pushing. It’ll be a lot of trial and error at first, but we all start somewhere!
Halogenoalkanes
Halogenoalkanes are a homologous series of saturated carbon compounds that contain one or more halogen atoms. They are used as refrigerants, solvents, flame retardants, anaesthetics and pharmaceuticals but their use has been restricted in recent years due to their link to pollution and the destruction of the ozone layer.
They contain the functional group C-X where X represents a halogen atom, F,Cl, Br or I. The general formula of the series is CnH2n+1X.
The C-X bond is polar because the halogen atom is more electronegative than the C atom. The electronegativity decreases as you go down group 7 therefore the bond becomes less polar. Flourine has a 4.0 EN whereas iodine has a 2.5 EN meaning it is almost non-polar.
The two types of intermolecular forces between halogenoalkane molecules are Van Der Waals and permanent dipole-dipole interactions. As the carbon chain length increases, the intermolecular forces (due to VDWs) increase as the relative atomic mass increases due to more electrons creating induced dipoles. Therefore the boiling point of the halogenoalkanes increases since more forces must be broken.
Branched chains have lower boiling points than chains of the same length and halogen because the VDWs are working across a greater distance and are therefore weaker.
When the carbon chain length is kept the same, but the halogen atom is changed, despite the effect of the changing polar bond on the permanent dipole-dipole interactions, the changing VDWs have a greater effect on the boiling point. Therefore as RAM increases, the boiling point increases meaning an iodoalkane has a greater boiling point than a bromoalkane if they have the same carbon chain length.
Halogenoalkanes are insoluble or only slightly soluable in water despite their polar nature. They are soluble in organic solvents such as ethanol and can be used as dry cleaning agents because they can mix with other hydrocarbons.
Summary
Halogenoalkanes are saturated carbon compounds with one or more halogen atoms. Their general formula is CnH2n+1X, where X is a halogen. Their functional group is therefore C-X.
They are used as refrigerants, solvents, pharmaceuticals and anaesthetics but have been restricted due to their link to the depletion of the ozone layer.
C-X bonds are polar due to the halogen being more electronegative than the carbon. The polarity of the bond decreases down group 7.
Van der Waals and permanent dipole-dipole interactions are the intermolecular forces in halogenoalkanes.
When carbon chain length increases, boiling points increase due to RAM increasing and the number of Van Der Waals increasing too.
In branched halogenoalkanes, Van Der Waals are working across a greater distance therefore attraction is weaker and boiling points are lower than an identical unbranched chain.
When the halogen is changed, the boiling point increases down the group due to the effect of a greater RAM - more VDWs mean more intermolecular forces to break.
Halogenoalkanes are insoluble in water but soluble in organic solvents like ethanol.
Bonus: free radical substitution reactions in the ozone layer
Ozone, O3, is an allotrope of oxygen that is usually found in the stratosphere above the surface of the Earth. The ozone layer prevents harmful rays of ultraviolet light from reaching the Earth by enhancing the absorption of UV light by nitrogen and oxygen. UV light causes sunburn, cataracts and skin cancer but is also essential in vitamin D production. Scientists have observed a depletion in the ozone layer protecting us and have linked it to photochemical chain reactions by halogen free radicals, sourced from halogenoalkanes which were used a solvents, propellants and refrigerants at the time.
CFCs cause the greatest destruction due to their chlorine free radicals. CFCs – chloroflouroalkanes – were once valued for their lack of toxicity and their non-flammability. This stability means that they do not degrade and instead diffuse into the stratosphere where UV light breaks down the C-Cl bond and produces chlorine free radicals.
RCF2Cl UV light —> RCF2● + Cl●
Chlorine free radicals then react with ozone, decomposing it to form oxygen.
Cl● + O3 —> ClO● + O2
Chlorine radical is then reformed by reacting with more ozone molecules.
ClO● + O3 —-> 2O2 + Cl●
It is estimated that one chlorine free radical can decompose 100 000 molecules of ozone. The overall equation is:
2O3 —-> 3O2
200 countries pledged to phase of the production of ozone depleting agents in Montreal, leading to a search for alternatives. Chemists have developed and synthesised alternative chlorine-free compounds that do not deplete the ozone layer such as hydroflurocarbons (HFCs) like trifluromethane, CHF3.
SUMMARY
Ozone, found in the stratosphere, protects us from harmful UV light which can cause cataracts, skin cancer and sunburn.
Ozone depletion has been linked to the use of halogenoalkanes due to their halogen free radicals.
CFCs were good chemicals to use because they have low toxicity and were non-flammable. The fact they don’t degrade means they diffuse into the stratosphere.
Chlorine free radicals are made when CFCs are broken down by UV light.
These go on to react with ozone to produce oxygen.
Chlorine free radicals are then reformed by reacting with more ozone.
It is a chain reaction that can deplete over 100 000 molecules of ozone.
There is a 200 country ban on their use and scientists have developed alternatives like hydrofluorocarbons to replace them
Happy studying!
-
 sweetgladiatorfesival liked this · 2 years ago
sweetgladiatorfesival liked this · 2 years ago -
 amateurchemstudent reblogged this · 4 years ago
amateurchemstudent reblogged this · 4 years ago -
 amateurchemstudent liked this · 4 years ago
amateurchemstudent liked this · 4 years ago -
 shaoshuan liked this · 4 years ago
shaoshuan liked this · 4 years ago -
 the-procrastinators-account liked this · 4 years ago
the-procrastinators-account liked this · 4 years ago -
 supernovadragoncat reblogged this · 4 years ago
supernovadragoncat reblogged this · 4 years ago -
 leahuzumakisblog reblogged this · 4 years ago
leahuzumakisblog reblogged this · 4 years ago -
 neptunium134 liked this · 4 years ago
neptunium134 liked this · 4 years ago -
 fadingoperatorpaperprune liked this · 4 years ago
fadingoperatorpaperprune liked this · 4 years ago -
 mauro-2002 liked this · 4 years ago
mauro-2002 liked this · 4 years ago -
 stormygems liked this · 4 years ago
stormygems liked this · 4 years ago -
 reprobate69me reblogged this · 4 years ago
reprobate69me reblogged this · 4 years ago -
 sheisinspotlight liked this · 4 years ago
sheisinspotlight liked this · 4 years ago -
 withoutaconscienceorafilter liked this · 4 years ago
withoutaconscienceorafilter liked this · 4 years ago -
 meadowslark reblogged this · 4 years ago
meadowslark reblogged this · 4 years ago -
 nerdygirl2424 liked this · 4 years ago
nerdygirl2424 liked this · 4 years ago -
 alittleworldlywise liked this · 4 years ago
alittleworldlywise liked this · 4 years ago -
 cottonneko liked this · 4 years ago
cottonneko liked this · 4 years ago -
 femtopulsed liked this · 4 years ago
femtopulsed liked this · 4 years ago -
 bug-catcher-jecht liked this · 4 years ago
bug-catcher-jecht liked this · 4 years ago -
 technospaceviking liked this · 4 years ago
technospaceviking liked this · 4 years ago -
 poporing reblogged this · 4 years ago
poporing reblogged this · 4 years ago -
 weisstheiss reblogged this · 4 years ago
weisstheiss reblogged this · 4 years ago -
 incredibly-sarcastic-url liked this · 4 years ago
incredibly-sarcastic-url liked this · 4 years ago -
 symbiotic-science reblogged this · 4 years ago
symbiotic-science reblogged this · 4 years ago -
 dreadlockmimi liked this · 4 years ago
dreadlockmimi liked this · 4 years ago -
 marimbahoe liked this · 4 years ago
marimbahoe liked this · 4 years ago -
 nablah liked this · 4 years ago
nablah liked this · 4 years ago -
 thesilverpond liked this · 4 years ago
thesilverpond liked this · 4 years ago -
 rebellious-sardine reblogged this · 4 years ago
rebellious-sardine reblogged this · 4 years ago -
 hoofit49 liked this · 4 years ago
hoofit49 liked this · 4 years ago -
 icefire8521 reblogged this · 4 years ago
icefire8521 reblogged this · 4 years ago -
 rainbowscythe liked this · 4 years ago
rainbowscythe liked this · 4 years ago -
 hmfic247 reblogged this · 4 years ago
hmfic247 reblogged this · 4 years ago -
 reporting-from-the-nerd-cave reblogged this · 4 years ago
reporting-from-the-nerd-cave reblogged this · 4 years ago -
 envman64 reblogged this · 4 years ago
envman64 reblogged this · 4 years ago -
 envman64 liked this · 4 years ago
envman64 liked this · 4 years ago -
 experimentalbeing reblogged this · 4 years ago
experimentalbeing reblogged this · 4 years ago -
 letstalkabtchemistry reblogged this · 4 years ago
letstalkabtchemistry reblogged this · 4 years ago -
 letstalkabtchemistry liked this · 4 years ago
letstalkabtchemistry liked this · 4 years ago -
 sciencethot liked this · 4 years ago
sciencethot liked this · 4 years ago -
 silkairplane liked this · 4 years ago
silkairplane liked this · 4 years ago -
 sccfan4ever liked this · 4 years ago
sccfan4ever liked this · 4 years ago -
 scienceprofessorquotes reblogged this · 4 years ago
scienceprofessorquotes reblogged this · 4 years ago -
 ursaminorjim liked this · 4 years ago
ursaminorjim liked this · 4 years ago -
 smurfetterac reblogged this · 4 years ago
smurfetterac reblogged this · 4 years ago -
 smurfetterac liked this · 4 years ago
smurfetterac liked this · 4 years ago


