Curate, connect, and discover
Student - Blog Posts
Do I have an essay to do for tomorrow ? Yes. Was It annouced yesterday ? Yes. Do I have any ideas of what I am doing ? No clue.

SOLO.TEDDYHICKMAN. LINKS IN THE BIO 👆🏾 #entrepreneur #filmmaker #screenwriter #student https://www.instagram.com/p/ClaIFUSLsch/?igshid=NGJjMDIxMWI=



────❁───────❁────────❁──────❁────────❁────
Hey 🌸˚˖⋆, so lately I've been thinking of ways to romanticize my college experience and decluttering and re-organizing my digital space with Notion has been helping with this.
What is your favorite kind of aesthetic for Notion.
I have beef with the 1% that would defend cockroaches! 😡

Get it done out of the way :)
Executive dysfunction be like:
Me: Go to lunch with you on Tuesday? Oh, I can’t I’m busy on Tuesday. On Tuesday I have to send The Email.
Tuesday Morning:
Me: Okay! Gotta send The Email today.
Me: But first, breakfast and a little YouTube to get the day going.
Tuesday Noon:
Me: okay! Day has begun! But it’s lunchtime so I’d better get some lunch.
Tuesday 5 o’clock:
Me: it is now Too Late to send The Email
Logical brain: if you send it now anyway, they’ll see it in the morning.
Me: good point. I should write The Email
Me: *doesn’t write The Email*
Tuesday 11pm:
Me: Tuesday’s almost over! Gotta send That Email!
Me: *opens email, enters address and subject line*
Me: Whew! I started The Email, what an accomplishment! Bed time.
Me: *stressing about sending The Email for the rest of the night, gets no sleep* I’ll send it as soon as I wake up.
Wednesday 11pm:
Me: I have to send The Email.
Me: *opens email*
Me: *Closes email*
Me: *opens email*
Me: *Types “Dear so and so”*
Me: bed time!
Me: *wakes up in a cold sweat at 3 am* I can’t believe I haven’t sent that email!
Me: *Opens laptop, sends email*
Me:
Me: >:( Why was that so hard????
I thought of a poem, but I cannot pen its words down. They are too heavy to be carried out my mind. Too artistic and beautiful to ever escape the confines of my brain.

Beautiful youngness, I’m triying these new stile with simple background


Hey I’m a broke Highschool student trying to pay for all my beta trips 😭😭😭. Help would be appreciated 🤍🤍🤍
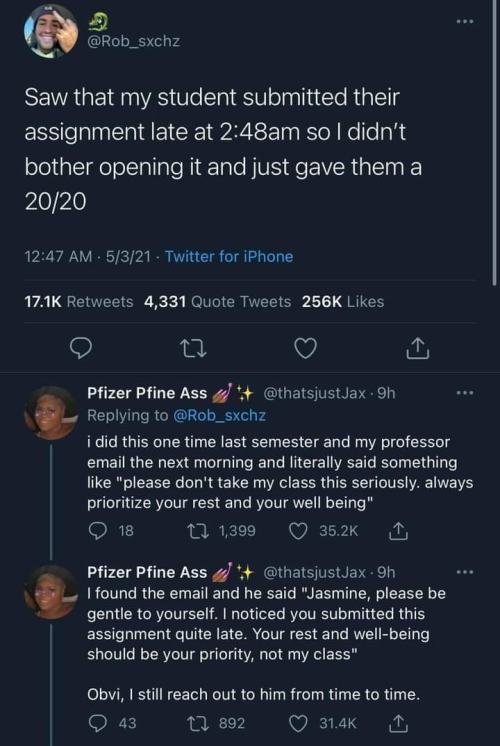
Sometimes my professors from university emailed my class, that they finished checking our last works or that they gave us final grades... One time it happened like: first group got they grades at 00:21 am, second group at 01:32 am, and third group, where I was, at 3:32 am... I don't think my professors care about our rest and well-being... Their class was always priority, even if the subject was dumb and we really don't need it
Finished 3 of 3 virtual classroom sessions now study to prepare for 1 of 8 course exams to earn my Certified Compensation Professional (CCP). I'm glad my employer is covering the cost.
Enthalpy - a thermodynamic property
When I first learned about enthalpy, I was shocked - it felt more like a physics lesson than a chemistry lesson. The thought of learning more about thermodynamics than my basic understanding from my many science lessons in lower school made me bored out of my mind. But enthalpy is actually pretty interesting, once you get your head around it…
Reactions which release heat to their surroundings are described to be exothermic. These are reactions like combustion reactions, oxidation reactions and neutralisation reactions. Endothermic reactions take in heat from their surroundings, such as in thermal decomposition. Reversible reactions are endothermic in one direction and exothermic in the other.
These facts are important when you start to look at enthalpy. Enthalpy is basically a thermodynamic property linked to internal energy, represented by a capital H. This is pretty much the energy released in bond breaking and made in bond making. We usually measure a change in enthalpy, represented by ∆H. ∆H = enthalpy of the products (H1) - enthalpy of the reactants (H2). This is because we cannot measure enthalpy directly.
In exothermic reactions, ∆H is negative whereas in endothermic reactions, ∆H is positive.
∆H is always measured under standard conditions of 298K and 100kPa.
In reversible reactions, the ∆H value is the same numerical value forwards and backwards but the sign is reversed. For example, in a forward exothermic reaction, the ∆H value would be -ve but in the backwards reaction (endothermic) the ∆H would be +ve.
Reaction profiles are diagrams of enthalpy levels of reactants and products in a chemical reaction. X axis is enthalpy rather than ∆H and the Y axis is the progress of reaction, reaction coordinate or extent of reaction. Two horizontal lines show the enthalpy of reactants and products with the reactants on the left and the products on the right. These should be labelled with their names or formulae.
In an endothermic reaction, product lines are higher enthalpy values than reactants. In an exothermic reaction, product lines are lower enthalpy values than reactants. The difference between product and reactant lines is labelled as ∆H. Values are measured in kJ mol-1.
Reaction pathways are shown with lines from the reactants to the products on enthalpy level diagrams. This shows the “journey” that the enthalpy takes during a reaction. They require an input of energy to break bonds before new bonds can form the products. The activation energy is the peak of the pathway above the enthalpy of reactants. It is the minimum amount of energy that reactants must have to react.

Standard enthalpy values are the ∆H values for enthalpy changes of specific reactions measured under standard conditions, represented by ⊖. There are three of these:
1. Standard enthalpy of reaction ( ΔHr⊖ )
The enthalpy change when substances react under standard conditions in quantities given by the equation for the reaction.
2. Standard enthalpy of formation ( ΔfH⊖ )
The enthalpy change when 1 mole of a compound is formed from its constitutent elements with all reactants and products in standard states under standard conditions.
The enthalpy of formation for an element is zero is it is in it’s standard state for example, O2 enthalpy is zero.
3. Standard enthalpy of combustion ( ΔcH⊖ )
The enthalpy change when 1 mole of a substance is burned completely in excess oxygen with all reactants and products in their standard states under standard conditions.
Values for standard enthalpy of formation and combustion must be kept to per mole of what they refer.
Summary
Reactions which release heat to their surroundings are described to be exothermic. Endothermic reactions take in heat from their surroundings, such as in thermal decomposition.
Reversible reactions are endothermic in one direction and exothermic in the other.
Enthalpy is a thermodynamic property linked to internal energy, represented by a capital H. We usually measure a change in enthalpy, represented by ∆H.
∆H = enthalpy of the products (H1) - enthalpy of the reactants (H2). We cannot measure enthalpy directly.
In exothermic reactions, ∆H is negative whereas in endothermic reactions, ∆H is positive.
∆H is always measured under standard conditions of 298K and 100kPa.
In reversible reactions, the ∆H value is the same numerical value forwards and backwards but the sign is reversed.
Reaction profiles are diagrams of enthalpy levels of reactants and products in a chemical reaction. They
In an endothermic reaction, product lines are higher enthalpy values than reactants. In an exothermic reaction, product lines are lower enthalpy values than reactants.
The difference between product and reactant lines is labelled as ∆H.
Values are measured in kJ mol-1.
Reaction pathways are shown with lines from the reactants to the products on enthalpy level diagrams. They plot enthalpy against reaction progress.
Reactions require an input of energy to break bonds before new bonds can form the products. The activation energy is the peak of the pathway above the enthalpy of reactants. It is the minimum amount of energy that reactants must have to react.
Standard enthalpy values are the ∆H values for enthalpy changes of specific reactions measured under standard conditions, represented by ⊖.
Standard enthalpy of reaction ( ΔHr⊖ ) is the enthalpy change when substances react under standard conditions in quantities given by the equation for the reaction.
Standard enthalpy of formation ( ΔfH⊖ ) is the enthalpy change when 1 mole of a compound is formed from its constitutent elements with all reactants and products in standard states under standard conditions.
The enthalpy of formation for an element is zero is it is in it’s standard state.
Standard enthalpy of combustion ( ΔcH⊖ ) is the enthalpy change when 1 mole of a substance is burned completely in excess oxygen with all reactants and products in their standard states under standard conditions.
Values for standard enthalpy of formation and combustion must be kept to per mole of what they refer.
Happy studying!
Covalent and Dative Bonds
Covalent and dative (sometimes called co-ordinate) bonds occur between two or more non-metals, e.g. carbon dioxide, water, methane and even diamond. But what actually are they?
A covalent bond is a chemical bond that involves the sharing of electron pairs between atoms. They are found in molecular elements or compounds such as chlorine or sulfur, but also in macromolecular elements and compounds like SiO2 and graphite. Covalent bonds are also found in molecular ions such as NH4+ and HCO3-.
Single covalent bonds have just one shared pair of electrons. Regularly, each atom provides one unpaired electron (the amount of unpaired electrons is usually equal to the number of covalent bonds which can be made) in the bond. Double covalent bonds have two shared pairs of electrons, represented by a double line between atoms, for example, O=C=O (CO2). Triple covalent bonds can also occur such as those in N ≡ N.
Dot and cross diagrams represent the arrangement of electrons in covalently bonded molecules. A shared pair of electrons is represented by a dot and a cross to show that the electrons come from different atoms.
Unpaired electrons are used to form covalent bonds as previously mentioned. The unpaired electrons in orbitals of one atom can be shared with another unpaired electron in an orbital but sometimes atoms can promote electrons into unoccupied orbitals in the same energy level to form more bonds. This does not always occur, however, meaning different compounds can be formed - PCl3 and PCl4 are examples of this.

An example where promotion is used is in sulfur hexafluoride (SF6). The regular configuration of sulfur atoms is 1s2 2s2 2p6 3s2 3p4. It promotes, as shown in the diagram (see excited state), two electrons: one from the 3s electrons to the 3d orbital and one from the 3p to the 3d. Therefore there are 6 unpaired electrons for fluorine atoms to join. It has an octahedral structure.

An atom which has a lone pair (a pair of electrons uninvolved in bonding) of electrons can form a coordinate bond with the empty orbital of another atom. It essentially donates an electron into this orbital which when formed, acts the same as a normal covalent bond. A coordinate bond therefore contains a shared pair of electrons that have come from one atom.

When ammonia reacts with a H+ ion, a coordinate bond is formed between the lone pair on the ammonia molecule and the empty 1s sub-shell in the H+ ion. An arrow represents the dative covalent bond (coordinate bond). Charges on the final ion must be showed.
Summary
A covalent bond is a chemical bond that involves the sharing of electron pairs between atoms. They are found in molecular elements or compounds as well as in macromolecular elements and compounds. Also found in molecular ions.
Single covalent bonds have just one shared pair of electrons. Double covalent bonds have two shared pairs of electrons, represented by a double line between atoms. Triple covalent bonds can also occur.
Dot and cross diagrams represent the arrangement of electrons in covalently bonded molecules. A shared pair of electrons is represented by a dot and a cross to show that the electrons come from different atoms.
Unpaired electrons are used to form covalent bonds - they can be shared with another unpaired electron in an orbital but sometimes atoms can promote electrons into unoccupied orbitals in the same energy level to form more bonds. This does not always occur, however, meaning different compounds can be formed.
An example where promotion is used is in sulfur hexafluoride (SF6).
An atom which has a lone pair (a pair of electrons uninvolved in bonding) of electrons can form a coordinate bond with the empty orbital of another atom.
It donates an electron into this orbital which when formed, acts the same as a normal covalent bond. A coordinate bond therefore contains a shared pair of electrons that have come from one atom.
When ammonia reacts with a H+ ion, a coordinate bond is formed between the lone pair on the ammonia molecule and the empty 1s sub-shell in the H+ ion. An arrow represents the dative covalent bond (coordinate bond). Charges on the final ion must be showed.
Halogenoalkanes
Halogenoalkanes are a homologous series of saturated carbon compounds that contain one or more halogen atoms. They are used as refrigerants, solvents, flame retardants, anaesthetics and pharmaceuticals but their use has been restricted in recent years due to their link to pollution and the destruction of the ozone layer.
They contain the functional group C-X where X represents a halogen atom, F,Cl, Br or I. The general formula of the series is CnH2n+1X.
The C-X bond is polar because the halogen atom is more electronegative than the C atom. The electronegativity decreases as you go down group 7 therefore the bond becomes less polar. Flourine has a 4.0 EN whereas iodine has a 2.5 EN meaning it is almost non-polar.
The two types of intermolecular forces between halogenoalkane molecules are Van Der Waals and permanent dipole-dipole interactions. As the carbon chain length increases, the intermolecular forces (due to VDWs) increase as the relative atomic mass increases due to more electrons creating induced dipoles. Therefore the boiling point of the halogenoalkanes increases since more forces must be broken.
Branched chains have lower boiling points than chains of the same length and halogen because the VDWs are working across a greater distance and are therefore weaker.
When the carbon chain length is kept the same, but the halogen atom is changed, despite the effect of the changing polar bond on the permanent dipole-dipole interactions, the changing VDWs have a greater effect on the boiling point. Therefore as RAM increases, the boiling point increases meaning an iodoalkane has a greater boiling point than a bromoalkane if they have the same carbon chain length.
Halogenoalkanes are insoluble or only slightly soluable in water despite their polar nature. They are soluble in organic solvents such as ethanol and can be used as dry cleaning agents because they can mix with other hydrocarbons.
Summary
Halogenoalkanes are saturated carbon compounds with one or more halogen atoms. Their general formula is CnH2n+1X, where X is a halogen. Their functional group is therefore C-X.
They are used as refrigerants, solvents, pharmaceuticals and anaesthetics but have been restricted due to their link to the depletion of the ozone layer.
C-X bonds are polar due to the halogen being more electronegative than the carbon. The polarity of the bond decreases down group 7.
Van der Waals and permanent dipole-dipole interactions are the intermolecular forces in halogenoalkanes.
When carbon chain length increases, boiling points increase due to RAM increasing and the number of Van Der Waals increasing too.
In branched halogenoalkanes, Van Der Waals are working across a greater distance therefore attraction is weaker and boiling points are lower than an identical unbranched chain.
When the halogen is changed, the boiling point increases down the group due to the effect of a greater RAM - more VDWs mean more intermolecular forces to break.
Halogenoalkanes are insoluble in water but soluble in organic solvents like ethanol.
Bonus: free radical substitution reactions in the ozone layer
Ozone, O3, is an allotrope of oxygen that is usually found in the stratosphere above the surface of the Earth. The ozone layer prevents harmful rays of ultraviolet light from reaching the Earth by enhancing the absorption of UV light by nitrogen and oxygen. UV light causes sunburn, cataracts and skin cancer but is also essential in vitamin D production. Scientists have observed a depletion in the ozone layer protecting us and have linked it to photochemical chain reactions by halogen free radicals, sourced from halogenoalkanes which were used a solvents, propellants and refrigerants at the time.
CFCs cause the greatest destruction due to their chlorine free radicals. CFCs – chloroflouroalkanes – were once valued for their lack of toxicity and their non-flammability. This stability means that they do not degrade and instead diffuse into the stratosphere where UV light breaks down the C-Cl bond and produces chlorine free radicals.
RCF2Cl UV light —> RCF2● + Cl●
Chlorine free radicals then react with ozone, decomposing it to form oxygen.
Cl● + O3 —> ClO● + O2
Chlorine radical is then reformed by reacting with more ozone molecules.
ClO● + O3 —-> 2O2 + Cl●
It is estimated that one chlorine free radical can decompose 100 000 molecules of ozone. The overall equation is:
2O3 —-> 3O2
200 countries pledged to phase of the production of ozone depleting agents in Montreal, leading to a search for alternatives. Chemists have developed and synthesised alternative chlorine-free compounds that do not deplete the ozone layer such as hydroflurocarbons (HFCs) like trifluromethane, CHF3.
SUMMARY
Ozone, found in the stratosphere, protects us from harmful UV light which can cause cataracts, skin cancer and sunburn.
Ozone depletion has been linked to the use of halogenoalkanes due to their halogen free radicals.
CFCs were good chemicals to use because they have low toxicity and were non-flammable. The fact they don’t degrade means they diffuse into the stratosphere.
Chlorine free radicals are made when CFCs are broken down by UV light.
These go on to react with ozone to produce oxygen.
Chlorine free radicals are then reformed by reacting with more ozone.
It is a chain reaction that can deplete over 100 000 molecules of ozone.
There is a 200 country ban on their use and scientists have developed alternatives like hydrofluorocarbons to replace them
Happy studying!

finally, some content! this was a quick info graphic I drew up on Procreate to revise for my ochem test tomorrow. disclaimer: I used information from this source (https://www.masterorganicchemistry.com/2010/05/24/imines-and-enamines/) since my own notes are based off lectures I received at my university that I’m not really allowed to share without heavy modification.
general post disclaimer: I’m an undergraduate student studying biochemistry and genetics. Posts are made for the purposes of education, revision and aesthetics. Not all the content I produce can be taken as entirely accurate and I do not take responsibility for errors made as a result of using this resource. Always consult course textbooks and lectures to aid in your specific learning outcomes. Do not repost without the original caption citing any extra references I used to make this post or remove my watermark. Other posts can be found on my blog as-studypeach@tumblr.com. Any problems, feel free to get in touch via my messages.

Help!!!
I need job ideas for James for my fic
Some context: past Jily who coparent Harry with Mary (marylily) , Harry goes to a coffee shop were he meets regulus (a barista) and matchmakes James and regulus
Other chapters jobs:
Lily - psychologist
Mary - bakery owner
Regulus - barista/photographer
Remus - phd literature student/assistant teacher
Im so desperate and open to all ideas!!
Read the fic here
If you have trouble remembering all the beef two historical figures had for your exam, just start shipping them.
I am not joking.
They hated each other before their coalition? Enemies to lovers. One of them was assassinated? Right person wrong time. They have portraits/photos together? They must’ve fought the urge to hold hands.
You’ll be surprised by how easy their lore becomes to remember
Hey, can you tell my mom the same thing? She won't let me read at night😐. Says, I should wake up at 4 AM instead, but that’s when I go to sleep!
Consistent study each night will help keep things fresh in your memory.


When we all scroll through Tumblr pinterest Instagram etc we all see tidy desks, beautifully written lessons, revision sessions in nice cafes. But can we show the chaos that revision often represents? While romanticising our studies is enjoyable and can even be motivating, it's important to remember that most people don't work like that and still succeed. So here's my revision chaos 4 days before the start of my exams: binders everywhere, even on the floor, barely legible sheets of rough paper and my flat untidy due to lack of time. Yes, the greatest difficulty in studying is the lack of time, so don't focus on the aesthetics of your lessons, but above all on understanding them and making them interesting.

I guess Cesar doesn’t like geology…

I've been working on a research project for a few months and I've just sent my first results to my teachers. I'm very proud of myself but also very stressed about their opinions.
It's the first project I've done on my own, so it's far from perfect, but it's a start.
My biggest flex ? Using Saltburn and quotes Jacob Elordi in my french essay 🫡

Gradually falling back in love with studying 🫶
Doing organic chemistry always helps me enjoy what I study
I'm going to one of the most prestigious schools in my country. I don't want to go there because I like the studies or because I'll be well paid when I leave. Not just because there's a ball where you can carry a real sword (need I ask for a better reason?).




7 hours of work today, my eyes are starting to sting but at least I've been productive (which proves that working with stress is effective).
J-5 before exams

Exam season = chaos


(Actually I have exams every weeks so it’s always chaos)
Remember when I said in high school that I wanted to do a difficult degree to push my limits? I take back everything I said, let me sleep and have a social life.











